Spotlight on Rare Diseases: How Data Diversity Drives Innovation in Rare Diseases Trials
In the world of rare disease research, data is everything, but diversity within that data is what makes it meaningful. Across the U.S., rare diseases collectively impact around 30 million people, or roughly 1 in 10 Americans. For conditions like Myasthenia Gravis (MG), an autoimmune disorder affecting approximately 60,000 people in the U.S., the importance of racially, ethnically, and geographically inclusive datasets cannot be overstated. In a space where patient populations are already limited, ensuring broad representation is a clinical necessity.
It's a timely reminder that rare disease trials demand more than scientific innovation; they require operational agility. This is where the story shifts from challenge to opportunity: a tech-enabled, data-diverse approach can drive not only compliance but faster, more inclusive, and more effective clinical outcomes. As we observe Myasthenia Gravis Awareness Month, this focus on MG prompts us to revisit a rapidly growing area: Immunology diseases, a complex and booming market. For deeper insights on how automation is reshaping immune disorder trials, check out our detailed Immunology Spotlight blog.
Diversity in clinical trials is a growing area of focus for regulatory bodies and the industry as a whole. Yet, many rare disease trials across the U.S. continue to face a critical shortfall in representation. When it comes to rare disease trial activity, oncology leads the way, accounting for 72% of rare disease clinical trials, while immunology, covering autoimmune and inflammatory conditions, represents the second-largest area at 10%. According to the FDA, less than 20% of participants in rare disease trials are from racially and ethnically diverse backgrounds. The consequences of this are significant such as skewed clinical insights, reduced generalizability of findings, and potential delays in regulatory approval.
Over the past five years, orphan drugs have accounted for over 50% of new active substance approvals in the U.S. and 45% in Europe. Of 268 drug launches in the U.S., 143 (53%) were for orphan-designated drugs. Rare diseases accounted for 44% of global clinical trial activity as of 2024, yet 95% of the 7,000–10,000 known rare diseases still lack approved treatments, underscoring a significant unmet need and ongoing focus for innovation.
This growing momentum has reshaped dealmaking dynamics in rare diseases. Large pharma companies have not just acquired entire rare disease portfolios, they also selectively targeted high-potential pipeline assets and marketed products from small-to-mid-sized biotechs (SMIDs) or specialized CROs. This strategic diversification reflected a clear industry signal: rare disease programs have become increasingly valuable across all stages of development.
For instance, AstraZeneca’s Alexion acquired a portfolio of preclinical gene therapies for rare diseases from Pfizer in 2023, in a deal worth up to $1 billion, showcasing confidence in early-stage innovation. On the other hand, Amgen’s $27.8 billion acquisition of Horizon Therapeutics brought in a mix of marketed products such as Tepezza (thyroid eye disease), Uplizna (neuromyelitis optica spectrum disorder), and Krystexxa, affirming the growing commercial value of mature rare disease assets.
These strategic moves reflect a broader industry realization: rare diseases offer not only therapeutic promise but also strong commercial potential. However, tapping into this potential is no easy task. The rare disease market remains deeply complex and fragmented, with thousands of conditions affecting small, dispersed populations. This makes standardized trial designs difficult and safety signal detection more challenging.
To address this, Real-World Data (RWD) plays a pivotal role by enabling access to diverse, decentralized data sources. It helps identify rare patient populations, uncover infrequent adverse events, and assess treatment outcomes in real-world settings. In diseases where clinical trial participation is often limited, RWD strengthens safety monitoring and improves regulatory readiness.
Making Rare Disease Trials More Inclusive: What’s Standing in the Way?
In the race to develop treatments for rare diseases, ensuring data diversity in clinical trials isn't just a regulatory box to check; it's a scientific and ethical necessity. Without inclusive representation, we risk creating therapies that don’t work for everyone or, worse, that exclude the very patients who need them most. Yet, the road to equitable participation in rare disease research is riddled with challenges.
Here are the key factors holding us back:
Data Source and Quality Issues
Recruitment and analysis are often hindered by poor data quality and fragmented sources. Datasets may lack completeness, standardization, or privacy safeguards and frequently fail to reflect target populations. Additionally, data silos across institutions and countries limit collaboration and aggregation essential for diverse rare disease trials.Regulatory and Protocol Constraints
While regulatory agencies stress the need for participant diversity, practical implementation remains difficult due to strict protocols. Eligibility criteria, aimed at controlling variability, can unintentionally exclude diverse patients, creating a paradox where data integrity efforts conflict with inclusive research goals in rare, complex conditions.Small and Heterogeneous Populations
Rare diseases affect small patient populations, making clinical trial recruitment inherently challenging. Enrolling a sufficiently large cohort is difficult, and achieving demographic diversity is even harder. Disease heterogeneity variations in symptom presentation and progression add complexity to recruitment and hamper reliable data interpretation.Underrepresentation of Minority Groups
Rare disease clinical trials often underrepresent minority groups, including racial and ethnic populations, older adults, people with disabilities, and those from lower socioeconomic backgrounds. This underrepresentation leads to inequitable access to new therapies and creates gaps in understanding treatment efficacy and safety across diverse populations.Logistical and Socioeconomic Barriers
Participants from underrepresented backgrounds often face logistical and socioeconomic barriers to trial participation, including limited access to trial sites, financial challenges, low awareness, and inadequate outreach. Additionally, geographic distance and centralized research at specialized centers further restrict inclusion from remote or underserved communities.
Cloud Concinnity: Centralizing Data for Equitable and Agile Rare Diseases Trials
Cloud Concinnity isn’t just a platform; it’s a purpose-built engine for transforming how rare disease trials are run. In a space where patient populations are small, site networks are dispersed, and timelines are unforgiving, Cloud Concinnity delivers the precision, visibility, and speed that today’s sponsors demand.
What sets Cloud Concinnity apart?
True Centralization, Not Just Integration: Our platform brings all critical oversight functions, diversity tracking, protocol changes, and risk logs into a single source of truth. No more toggling between systems or waiting on outdated reports.
Built for Cross-Functional Agility: Cloud Concinnity is uniquely designed to align sponsors, CROs, investigators, and oversight boards in one collaborative environment, enabling rapid decisions without compromising compliance.
Regulatory Intelligence Built In: With automated audit trails, protocol deviation tracking, and inspection-ready documentation, teams stay aligned with evolving FDA and EMA expectations without scrambling before submissions.
In rare disease R&D, there’s no room for rework. Cloud Concinnity gives sponsors the oversight they need and the foresight they’ve never had, ensuring every patient, site, and data point contributes to progress.
Discover how Cloud Concinnity can help make your next rare disease trial more inclusive, efficient, and successful.
Spotlight on Immunology: Why Automation Is the New Cure for Immune Disorder Trials
Immunological diseases represent one of the most complex and rapidly evolving areas in medicine today. From widespread conditions like rheumatoid arthritis and psoriasis to rarer, debilitating illnesses such as Myasthenia Gravis (MG), these disorders collectively affect millions of people worldwide and pose significant challenges in diagnosis, treatment, and long-term management.
In the US alone, more than 15 million people live with autoimmune diseases, highlighting the urgent need for more targeted and efficient approaches. Increasingly, the response is being shaped by Real-World Data (RWD) and automation-driven powerful tools that are helping pharma companies, researchers, and regulators rethink how immunology trials are designed, conducted, and applied in real-world settings. With June marking Myasthenia Gravis Awareness Month, it’s a timely moment to spotlight the broader immunology landscape while also examining how the industry is embracing data-driven innovation to improve outcomes.
The Rise of Immunology Trials: Why Smarter Tools Matter
Immunology trials are evolving rapidly. As of 2025, there are currently 6,373 ongoing clinical trials involving immunology worldwide, highlighting immunology as a significant but niche area within the broader clinical research landscape. Despite representing a smaller percentage of global trials, the rapid growth in immunology research reflects the increasing demand for innovative solutions to manage complex immune-related diseases.
Of these, 1,992 trials are in Phase III and 1,923 are in Phase II, highlighting the push toward more advanced and targeted therapies. This surge underscores the growing demand for innovative solutions to manage complex immune-related diseases and streamline trial operations efficiently.
The landscape of immunology clinical trials is broad, reflecting the complexity and diversity of immune-related diseases. In 2025, immunology clinical trials span autoimmune diseases, immuno-oncology, infectious diseases, allergies, rare immune disorders, and transplantation. Autoimmune and cancer immunotherapies lead the trial volume. Rare diseases and transplant-related trials explore novel gene and cell therapies, reflecting the field’s shift toward precision, innovation, and diverse immune-targeting strategies across all trial phases.
Driving the growth of immunology clinical trials is a robust ecosystem of industry leaders and research institutions committed to advancing immune-related therapies. At the forefront are major sponsors supporting a wide range of studies across therapeutic areas. Johnson & Johnson leads as the top sponsor of ongoing trials, followed by Bristol-Myers Squibb, Novartis, Assistance Publique – Hôpitaux de Paris, and the National Institute of Allergy and Infectious Diseases, all playing a pivotal role in accelerating breakthroughs in immunology.
But progress isn’t without challenges:
Immune diseases are heterogeneous, making trial design and interpretation complex.
Patient recruitment is often slow, and dropout rates remain high.
This is where automation steps in as a true game-changer. Cloud Concinnity is revolutionizing clinical trial management by bringing together investigators, sponsors, and data in a single, transparent hub. This centralized oversight not only accelerates decision-making but also enhances collaboration, ensuring that trials stay on track and patients receive the best possible care throughout the study.
Effectively Harnessing the Power of Diverse Data: Streamlining Complexity and Enhancing Safety in Immunology Trials
Immunology trials generate an immense and varied volume of data, from clinical endpoints and patient-reported outcomes to genomic profiles and real-world evidence. While this diversity provides a comprehensive understanding of disease progression and treatment effects, it also presents a major challenge: how to effectively integrate, standardize, and analyze this heterogeneous data to ensure both operational efficiency and patient safety.
Central to this challenge is the management of safety-related data, which is critical given the complex and often unpredictable immune responses seen in immunology patients. Adverse events can be varied and sometimes rare, making early detection and real-time monitoring essential to protect patients and comply with stringent regulatory standards.
Cloud Concinnity centralizes and streamlines clinical trial data, providing stakeholders with real-time visibility and automated compliance monitoring. This supports proactive safety oversight, enabling early detection of adverse events, facilitating adaptive trial management, and helping teams maintain regulatory alignment with frameworks such as the FDA’s Real-World Evidence Program.
In sum, leveraging advanced technologies and automation to streamline diverse immunology data not only reduces operational complexity but also plays a vital role in safeguarding patient health, accelerating the path to safer, more effective immunotherapies.
From Data Chaos to Trial Clarity: Immunology at the Speed of Cloud Concinnity
Cloud Concinnity is uniquely positioned to centralize data, align stakeholders, and ensure compliance, especially as pharma companies strive to move faster without sacrificing rigor. As we shine a spotlight on Myasthenia Gravis this June, it's clear that the future of immunology isn’t just about new drugs; it's about smarter data, stronger partnerships, and more integrated decision-making. Whether you're a trial sponsor, CRO, or oversight committee member, now is the time to ask: Are your tools built for the complexity of immunology?
That’s where Cloud Concinnity’s 3Cs Framework and built-in automation come in:
Centralization: Cloud Concinnity brings all critical trial functions into one secure, cloud-based platform. From protocol amendments to oversight documentation and regulatory workflows, everything is organized in a single source of truth, streamlining trial operations and ensuring every stakeholder is working from the same playbook.
Collaboration: Immunology trials involve cross-functional teams from sponsors and CROs to immunologists and site investigators. Cloud Concinnity enables real-time access, updates, and decision-making to break down silos and keep trials moving with agility, even as complexity scales.
Compliance: In a field where patient safety and regulatory alignment are non-negotiable, our platform’s built-in compliance tools offer peace of mind. Automated risk alerts, audit trails, and dynamic dashboards help teams stay ahead of challenges while maintaining audit readiness at every stage.
See how Cloud Concinnity helps streamline immunology trials, centralized, compliant, and built for real-time collaboration through the power of automation.
Spotlight on Neurology: Strategic Shifts Powering the Future of Mental Health Trials
Mental health is a critical public health issue worldwide, with nearly 1 in 5 adults affected by anxiety, depression, bipolar disorder, or schizophrenia in the US alone. (NIMH). Across the US, awareness of mental health conditions has increased due to advocacy, education, and challenges amplified by the COVID-19 pandemic. The demand for effective, accessible treatment options is surging. As the stigma declines and more individuals seek help, the pressure on healthcare systems and pharmaceutical companies to respond with innovation has never been greater.
As we observe Mental Health Awareness Month, let’s take a moment to reflect on how increased investments in clinical trials drive innovation in mental health treatment and improve the lives of millions of people. Pharma companies are stepping up with robust R&D investments into new therapies for depression, anxiety, schizophrenia, and neurodegenerative diseases. As of September 2024, ClinicalTrials.gov listed 360 biotech-sponsored anxiety trials in North America and 121 in Europe. Companies like AstraZeneca, Novartis, Pfizer, and Eli Lilly are leading this charge. In the US, a transparent FDA regulatory framework, combined with funding from industry, academia, and government, is fueling innovation at scale.
Consumer demand for better treatments is reflected in drug revenue numbers as well as invested research dollars. Roche’s Ocrevus (Ocrelizumab) led the multiple sclerosis segment with $7.4 Bn in 2024 global sales. Janssen’s Invega franchise, including Invega Sustenna, brought in $4.2 Bn globally, with $3.1 Bn (76%) from the US. AbbVie’s Vraylar (Cariprazine) continues to gain traction for bipolar disorder and schizophrenia, and Takeda’s Vyvanse (Lisdexamfetamine) remains a leading choice for ADHD and binge eating disorder. As mental health gains mainstream focus, these drugs are shaping a more inclusive and proactive care model.
How the Industry is Stepping Up to Solve the Mental Health Crisis
Pharmaceutical companies are no longer limiting their role in mental health to pill-based solutions. With rising mental illness prevalence and a fast-evolving digital ecosystem, pharma is adopting a multi-dimensional approach to close treatment gaps and improve outcomes. Here’s how they’re making it happen:
Precision Psychiatry: From One-Size-Fits-All to Personalized Care
Traditional antidepressants and antipsychotics often fail to address the biological diversity among patients. In 2025, pharma is shifting toward biomarker-driven psychiatry, leveraging neuroimaging, genetics, and machine learning to create tailored therapies. Janssen, for instance, is advancing this approach in treatment-resistant depression through the biomarker-supported use of Esketamine (Spravato).
2. Going Beyond Pills: Digital Therapeutics and Hybrid Care Models
Pharma companies are expanding their offerings through digital therapeutics (DTx), which are FDA-cleared software treatments that complement medication. Novartis, through its collaboration with Pear Therapeutics, has introduced prescription digital cognitive behavioral therapy tools to be used alongside pharmacological treatments for serious mental illness.
3. AI and Digital Transformation in Clinical Trials
Artificial Intelligence is enabling pharma companies to run smarter, faster, and more inclusive clinical trials by identifying ideal participants, optimizing trial designs, and predicting treatment efficacy. Companies like Pfizer are embracing decentralized models for mental health trials through platforms such as TrialSpark and Medable, making remote recruitment and monitoring a reality.
4. Prioritizing Access and Equity
Addressing systemic disparities, pharma is embedding equity into trial design and patient engagement, especially for underserved racial and rural populations. Alkermes is leading by example, conducting real-world evidence programs in community-based settings to enhance access to Lybalvi, a treatment for schizophrenia.
Pharma’s Strategic Approaches for Successful Mental Health Trial Innovation
Fostering Cross-Sector Collaboration: Successful clinical trials increasingly rely on collaborative models that involve multiple stakeholders from the outset. Bringing together researchers, regulators, CROs, and patient groups early in trial planning leads to stronger study design and smoother execution, enriched by diverse perspectives.
Leveraging Emerging Technologies for Study Design & Execution: Integrating cutting-edge technology, such as AI, machine learning, and digital tools, is transforming how clinical trials are conducted. These tools enable real-time data analysis, remote monitoring, and adaptive trial designs, enhancing efficiency and accelerating decision-making.
Optimizing Recruitment & Retention: Participant recruitment and retention remain key challenges in clinical trials. To address these, sponsors are adopting patient-centric strategies such as personalized communication, flexible visit schedules, and digital engagement platforms, preserving trial integrity and data quality.
Digital Transformation Initiatives: Pharma leaders are prioritizing digital transformation through investments in cloud-based platforms, automated trial oversight tools, and integrated data systems. Cloud Concinnity exemplifies this shift, centralizing trial operations, ensuring compliance, and scaling with ease. These innovations reduce operational friction and enable real-time visibility across stakeholders, ultimately speeding up development timelines.
Strategic Collaborations & Partnerships: Pharmaceutical companies are increasingly engaging in strategic partnerships with academic institutions, biotech startups, and technology providers. These collaborations allow for knowledge exchange, risk-sharing, and co-development of innovative solutions, particularly in mental health and neurology.
Geographic Expansion: Global market expansion remains a top priority for pharma companies aiming to broaden trial diversity and market reach. By establishing a presence in emerging regions, companies can access more varied patient populations, improve inclusivity in research, and accelerate regulatory approvals. Expanding clinical trial networks globally also allows for quicker enrollment and more robust data collection.
Cloud Concinnity’s 3Cs Framework: Powering Trial Transformation
Innovation driven clinical trials demand agility, compliance, and seamless coordination across diverse teams. Cloud Concinnity’s unified platform is designed to meet these needs simplifying operations, reducing risk, and accelerating innovation.
Here’s how we support trial transformation:
Centralization: Cloud Concinnity offers a secure, cloud-based workspace that centralizes all essential trial operations. From managing documents and approvals to handling regulatory workflows, everything is streamlined in one place, ensuring efficiency, consistency, and accessibility across teams.
Collaboration: With real-time access and communication tools, Cloud Concinnity enables seamless coordination between sponsors, CROs, and research teams. This effortless collaboration keeps trials aligned, minimizes delays, and fosters a more agile trial environment.
Compliance: Proactive risk & compliance monitoring is built into the platform, with automated tools that flag issues early helping teams stay ahead of regulatory demands without the added burden. Dynamic dashboards and reports provide actionable insights, enabling faster, data-driven decisions that keep trials compliant and moving forward.
Click here to see how Cloud Concinnity can help your next mental health trial succeed.
Spotlight on Oncology: Accelerating Breakthroughs in Early-Phase Oncology Trials
April marks National Cancer Control Month in the US, a time dedicated to increasing awareness of cancer prevention, early detection, and advancements in treatment. Cancer remains a leading health challenge in the United States, with nearly 2 million new cases and over 600,000 deaths projected in 2024. However, scientific progress and cutting-edge innovations in oncology research are driving faster, more effective treatments, offering renewed hope to patients and their families.Early-phase oncology trials are pivotal in this transformation, serving as the foundation for novel therapies that could redefine cancer care. With the integration of digital technologies, these trials are becoming more efficient, data-driven, and patient-centric, accelerating the journey from discovery to treatment.
This article explores how emerging innovations and cloud technology are revolutionizing early-phase oncology trials accelerating breakthroughs, optimizing operations, and enhancing collaboration for more efficient and impactful research.
Innovations and Emerging Trends in Oncology Trials
The landscape of oncology clinical trials is evolving rapidly. Some key trends shaping the future include:
Leveraging Real-World Evidence (RWE): Real-world data provides valuable insights into patient characteristics, treatment patterns, and outcomes beyond controlled clinical trial settings. Utilizing this information helps design studies that better reflect real-world clinical practice, improving their relevance and feasibility.
Implementing Adaptive Trial Designs: These trial frameworks allow for modifications to study protocols, such as adjusting dosage levels or expanding participant groups based on interim results. Adaptive designs are particularly beneficial in oncology, where treatment responses can vary significantly across different patient populations.
Expanding Decentralized and Hybrid Trial Approaches: By integrating virtual components and remote monitoring technologies, trials can become more accessible and inclusive while maintaining scientific integrity.
Advancing Precision Medicine in Clinical Trials: Modern studies increasingly focus on specific genetic or molecular markers, requiring expertise in biomarker identification and patient stratification. Incorporating advanced diagnostic and monitoring technologies ensures that trials are equipped to meet these evolving demands.
Artificial Intelligence (AI) and Machine Learning (ML) Integration: AI and ML are transforming oncology trials by enabling rapid data analysis, predictive modeling, and enhanced patient selection. These technologies help identify cancer earlier and more accurately through biomarker and imaging analysis.
Digital Medicine: Digital medicine improves oncology trials by enabling real-time patient monitoring and data collection. It enhances disease assessment, treatment response tracking, and patient accessibility while eliminating geographical barriers.
The Future of Early Cancer Detection
The landscape of early cancer detection is rapidly evolving, driven by groundbreaking technologies that enhance early-stage diagnosis, personalized screening, and targeted treatments. These innovations are set to redefine cancer care by enabling earlier intervention, improving patient outcomes, and optimizing clinical workflows. As advancements in digital health, AI-powered diagnostics, and precision medicine continue to shape oncology research, the integration of these technologies into early-phase trials will play a crucial role in accelerating the development of life-saving therapies.
How Cloud Concinnity is Reshaping Early-Phase Oncology Trials
Emerging technologies are revolutionizing early cancer detection, enabling advanced diagnosis, personalized screening, and targeted treatments improving patient outcomes and streamlining clinical workflows. Technology is revolutionizing early-phase oncology trials, addressing the complexities of trial oversight, data management, and regulatory compliance. Cloud Concinnity’s SaaS platform centralizes trial operations, automates compliance workflows, and leverages AI-powered analytics to enhance efficiency. With the latest advancements in Cloud Concinnity® 3.3.0, we’re introducing streamlined coordination tools, enhanced security features, and smarter staffing transition management, ensuring oncology trials run seamlessly while accelerating breakthroughs in cancer research.
Click here to learn more and see how these updates can optimize your oncology trial management. If you'd like to experience these enhancements firsthand, click here to request a demo.
Let’s continue advancing clinical trial innovation, ensuring that life-saving cancer therapies reach patients faster. By refining trial processes and embracing cutting-edge technology, we can accelerate the future of oncology research
Spotlight on Breast Cancer: Tackling Regulatory Compliance Challenges and Unlocking Opportunities
Breast cancer remains the most prevalent cancer worldwide, with approximately 2.3 million new cases diagnosed each year. In the US, about 1 in every 8 women will be affected by breast cancer in her lifetime [1]. As researchers push the boundaries of innovation to develop better treatment options, clinical trials play a pivotal role in advancing groundbreaking therapies. However, navigating the regulatory landscape of breast cancer trials is a complex process. These trials must adhere to rigorous regulatory frameworks to ensure patient safety, data integrity, and scientific validity and must comply with strict regulatory frameworks to ensure patient safety, data integrity, and scientific validity. Ensuring compliance with these stringent regulatory requirements remains a significant hurdle, yet it is crucial for the success of these trials.
This article delves into the challenges faced in regulatory compliance of breast cancer trials, explores opportunities to enhance the process, and highlights how we support efficient and compliant clinical trial oversight.
Recent Innovations in Breast Cancer Clinical Trials
Recent innovations in breast cancer clinical trials focus on improving detection, diagnosis, monitoring, and treatment using new technologies, drug combinations, and personalized approaches driven by clinical trials. Some key innovations include:
Immunotherapy in Triple-Negative Breast Cancer (TNBC): A major study is evaluating whether some patients can stop immunotherapy after surgery if all signs of cancer are gone. This could help reduce side effects while still preventing the cancer from coming back[2].
Label expansion for Enhertu (trastuzumab deruxtecan): Enhertu was approved for HER2-positive breast cancer, but this new indication extends its use to patients with HER2-low and HER2-ultralow metastatic breast cancer also, a group that previously had limited targeted treatment options[3].
Precision Medicine for Breast Cancer: New treatment combinations are making cancer care more personalized. These include special drugs that target cancer cells in different ways, improving treatment for patients with tough-to-treat breast cancers like TNBC[4].
Tumor Profiling: Doctors are using tumor profiling, looking for certain genes within the tumor to help select the most appropriate treatment path. Looking at the genetic profile of the tumor can help predict whether the cancer is likely to reoccur or metastasize and whether chemotherapy is recommended in patients with early-stage, hormone receptor-positive breast cancer[5].
3-D Mammography The Tomosynthesis Mammographic Imaging Screening Trial (TMIST) compares the number of advanced cancers detected in women screened with 3-D mammography to those detected in women screened with 2-D mammography[5].
New Imaging Tests: Newer types of tests are being developed for breast imaging, including Scintimammography (molecular breast imaging), Positron emission mammography (PEM), Electrical impedance imaging (EIT), and Elastography[5].
Challenges in Regulatory Compliance for Breast Cancer Trials
Breast cancer clinical trials face numerous regulatory hurdles that can delay drug approvals which further increase costs, and impact patient access to innovative treatments. So let’s take a closer look at these challenges.
Regulatory Barriers and Approval Delays: Differences in regulatory requirements between agencies like the US FDA and the EMA can lead to approval delays due to a lack of harmonization, making clinical trials more expensive and posing significant financial barriers. This, in turn, makes it increasingly challenging to bring new drugs to market[6].
Post-Approval Change Management: Managing changes post-approval is crucial but faces regulatory obstacles, such as issues with dossier preparation and data[6].
Surrogate vs. Event Endpoints: Surrogate endpoints in oncology allow faster assessments and FDA approval but require careful validation. Without follow-up studies, their correlation with survival may be uncertain[6].
Opportunities in Regulatory Compliance for Breast Cancer Trials
As breast cancer research advances, regulatory frameworks must evolve to keep pace with innovation. Streamlining compliance processes can accelerate approvals, improve global collaboration, and enhance patient access to breakthrough therapies. Let’s explore some key opportunities shaping the future of regulatory compliance.
Harmonization of Regulatory Requirements: Harmonizing regulatory requirements across different countries can reduce duplication of effort for pharmaceutical companies, increase efficiency, improve access to innovative treatments, and facilitate global trials[6].
Agile and Responsive Regulation: Regulators can adopt risk-proportionate approaches to reviewing clinical trial applications, which can expedite the decision-making process[7].
Leveraging Cloud-Based Platforms for Regulatory Management: Cloud-based platforms offer an accessible solution for managing data, streamlining workflows, and enhancing collaboration. However, Cloud Concinnity goes beyond protecting sensitive data, training users, and managing studies. It streamlines clinical oversight with unified account management, controlled document collaboration, centralized alerts, compliance monitoring, automated audits, flexible workflows, and adaptive pricing.
Usage of Accelerated Approval Pathways: Utilizing accelerated approval pathways with predefined surrogate endpoints can help avoid delays in the approval of innovative breast cancer therapies[6].
Real-World Data (RWD) Integration: Utilizing real-world data alongside clinical trial data can strengthen regulatory submissions, demonstrating the real-world effectiveness of new treatments.
Cloud Concinnity’s Unified Platform: A Game Changer
Navigating regulatory compliance in breast cancer clinical trials is a challenging yet rewarding endeavor. Adopting cutting-edge technologies and strategic approaches can transform compliance into a competitive advantage as the landscape evolves with new innovations and global harmonization efforts. At Cloud Concinnity, we are committed to driving efficiency, transparency, and innovation in clinical trial oversight.
Here’s how we can help:
Centralized and Compliant Hub: Our cloud-based platform offers a secure, unified space for managing trial documentation, approvals, and regulatory updates.
Facilitated Access & Collaboration: We provide seamless access to essential documents, enabling teams to collaborate effectively while maintaining compliance.
Automated Risk Mitigation: Our system automates compliance tracking, reducing the likelihood of regulatory breaches and improving trial efficiency.
Enhanced Transparency & Reporting: With real-time dashboards and automated reporting, Cloud Concinnity ensures that trial sponsors and regulatory bodies have full visibility into
Click here to explore how Cloud Concinnity can help your breast cancer trial meet regulatory standards with ease.
Spotlight on Cardiology: Advancements and Clinical Trial Implications
Heart disease has remained the leading cause of death in the U.S. for over 100 years, as highlighted by the 2024 American Heart Association's heart disease and stroke statistics. As we observe American Heart Month, the crisis continues to impact many communities. However, advancements in cardiology are bringing new hope for better heart care. Innovations such as gene and cell therapies, next-generation anticoagulants, and minimally invasive procedures are enhancing heart function, reducing hospital stays, and personalizing care for improved patient outcomes.
In this article, we explore key breakthroughs in cardiology and how technology is driving these advancements forward.
The following innovations are reshaping the treatment of heart disease and offering new hope for patients:
1. Gene and Cell Therapies
Emerging gene and cell therapies are paving the way for more targeted treatments, offering potential long-term solutions for heart failure and myocardial infarction. These therapies hold promise for repairing damaged heart tissue at the cellular level, potentially reversing some of the effects of heart disease.
2. Next-Generation Anticoagulants
Newer anticoagulants are providing safer and more effective options for preventing and treating thrombotic events. These medications carry fewer side effects compared to traditional blood thinners, offering more tailored approaches to managing blood clot risks.
3. Transcatheter Aortic Valve Replacement (TAVR)
This minimally invasive procedure offers a transformative treatment option for aortic valve stenosis. TAVR significantly reduces recovery time and is now widely used for both high- and low-risk patients, improving their quality of life with quicker procedures and fewer hospital stays.
4. SGLT2 Inhibitors for Heart Failure
Originally developed for type 2 diabetes, SGLT2 inhibitors have emerged as a key therapy for heart failure. These drugs not only reduce blood pressure and fluid retention but also improve cardiovascular outcomes, making them essential in managing heart disease.
5. Mitral Valve Clips (MitraClip)
This non-surgical device is used to treat mitral regurgitation by restoring proper valve function. MitraClip reduces hospitalizations and has shown to improve long-term survival for patients who are otherwise at high risk for surgery.
6. GLP-1 Agonists for Cardiovascular Health
Medications like Ozempic and Wegovy are now recognized for their dual role in regulating blood sugar and reducing cardiovascular risk. By promoting weight control and lowering the risk of heart attack and stroke, these drugs are becoming essential in managing cardiac health.
7. Advancements in Percutaneous Procedures
Innovations in angioplasty and stenting are enhancing blood flow and providing effective alternatives to open-heart surgery. These procedures not only reduce recovery times but also offer patients less invasive options for treatment.
The Future of Cardiology Innovation
The future of cardiology is shaped by advancements in biomaterials, nanotechnology, and precision medicine. These innovations are making treatments more effective and less invasive, but their integration into clinical practice requires evolving clinical trial methodologies. The growing complexity of treatments demands adaptive research frameworks to ensure safety and accessibility.
Adapting Clinical Trials for Emerging Therapies
As cardiology treatments advance, clinical trials must keep pace with more complex protocols. Today’s trials require precise biomarker tracking, selective patient recruitment, and the management of large-scale data. Additionally, stringent regulatory oversight necessitates meticulous documentation to comply with global standards. Addressing these factors is critical to accelerating the approval of innovative therapies.
Enhancing Clinical Trials with Technology
Technology plays a crucial role in addressing the evolving demands of cardiology clinical trials. Cloud Concinnity’s SaaS platform centralizes oversight, automates regulatory compliance, and leverages AI-powered analytics to improve trial efficiency. With the launch of Cloud Concinnity® 3.3.0, we're introducing several updates to further enhance clinical trial coordination. This latest version brings streamlined meeting scheduling, smarter tools for staffing transitions, and enhanced security features, including upgraded two-factor authentication.
Click here to see more and discover how these updates can optimize your trial management. If you'd like to experience these updates firsthand, click here to request a demo.
Let's continue building on these advancements, ensuring that innovative therapies are swiftly brought to patients. By refining clinical trial processes and embracing new approaches, we can accelerate the future of heart health.
Spotlight on Blindness: Novel Therapies and Implications for Clinical Trials
The fight against blindness has reached a pivotal moment. Advances in therapies are revolutionizing the treatment of diseases such as glaucoma and macular degeneration, offering new hope to millions worldwide. Diseases are now being addressed with innovative treatments that promise not only to halt progression but also to restore vision. These breakthroughs, while offering hope to patients, bring unique challenges and opportunities to the clinical trial landscape. Harnessing these breakthroughs demands not only cutting-edge science but also unparalleled efficiency in trial coordination.
Promising Therapies Addressing Leading Causes of Irreversible Blindness
Sustained-Release Drug Implants: Glaucoma, a leading cause of irreversible blindness, affects over 4 million people in the U.S. alone, with African Americans and Hispanic populations at higher risk. Characterized by optic nerve damage, glaucoma’s progressive nature, and, unfortunately, often delayed diagnosis, it requires timely intervention. Intraocular pressure (IOP) is the only known modifiable risk factor for glaucoma and thus the primary target for treatment. A component unique to glaucoma management is that a heavy portion of the burden is placed upon patients, with treatment response predicated on daily eyedrop therapy and compliance. Sustained-release drug implants can deliver consistent medication over months, significantly reducing variability in IOP as well as simplifying the treatment regimen for patients.
Intravitreal Medications: Nearly 20 million people in the United States are affected by macular degeneration. This disease notoriously affects central vision predominantly, making it particularly debilitating. The first anti-vascular endothelial growth factor therapy (anti-VEGF) was only approved for wet macular degeneration as recently as 2004. Since then, the sector has exploded with available intravitreal medications within this class of drugs, for use in both macular degeneration and diabetes. But only very recently, in 2023, was the first pharmacologic treatment for dry macular degeneration (a complement C5 protein inhibitor) approved for clinical use.
Gene and Stem Cell Therapies: Limitations of many current treatments across both the glaucoma and retina subspecialties are the duration of action (requiring redosing) as well as the failure to prevent vision loss (only to delay or slow). Simply put, treatments rather than cures. Because both glaucoma and macular degeneration have known hereditary components and genetic factors, solutions within this area are particularly compelling. While stem cell therapies are more commonly being utilized for childhood retinal disorders, they represent a promising treatment strategy across ophthalmic diseases.
Implications for Clinical Trials
These breakthroughs bring unique challenges to clinical trial design and execution. Here’s how they reshape the landscape:
Complex Protocols: Advanced therapies require multi-phase trials involving intricate endpoints, including visual acuity, IOP metrics, and biomarkers
Patient Recruitment: Identifying and enrolling participants with specific genetic profiles or disease stages necessitates targeted outreach and robust inclusion criteria
Data Complexity: Trials involving gene and stem cell therapies generate vast datasets, demanding sophisticated platforms for real-time collaboration and analysis
Regulatory Scrutiny: Novel therapies often face heightened regulatory requirements, necessitating meticulous documentation and compliance oversight
The Role of Technology in Clinical Trials
For industry leaders aiming to dominate the ophthalmology space, technology is a key enabler. Our SaaS platform transforms clinical trial coordination, offering:
Centralized Oversight: Seamlessly manage multi-site studies and track trial progress in real time.
Streamlined Collaboration: Facilitate secure, efficient communication among independent safety committees and Data Monitoring Committees (DMCs).
Regulatory Compliance: Automate documentation workflows to align with FDA and EMA guidelines, reducing administrative burdens.
Actionable Insights: Leverage AI-powered analytics to uncover trends, optimize protocols, and accelerate timelines.
A Call to Innovators
As we stand at the intersection of scientific discovery and technological innovation, the opportunity to redefine ophthalmology is clear. For innovators in this domain, investing in tools that enhance trial efficiency is as critical as advancing retinal therapies themselves. Cloud Concinnity can offer you a universal solution to streamline your clinical trial process by bringing you the 3Cs: Centralization - saves time by centralizing documentation activities, Collaboration - increases trial workflow efficiency by facilitating collaboration for key decision-making, and Compliance - ensures compliance to maintain quality in work.
By embracing this innovation, we can not only accelerate breakthroughs but also ensure they reach the patients who need them most. Together, let’s illuminate the path to a future where blindness is no longer irreversible.
2024 in Review: Transforming Clinical Trial Coordination
As the year draws to a close, we at The Concinnity Company are proud to celebrate an extraordinary 2024. Fueled by our commitment to address Centralization, Collaboration, and Compliance challenges in clinical trials, we’ve worked alongside the world’s largest CROs and sponsors to transform clinical trial oversight with process intelligence. This year has been a testament to our mission of streamlining processes and advancing clinical trial coordination.
Milestones That Defined 2024
Geometric growth in Platform Adoption: 250+% growth.
Our sponsor count surged by an impressive 260%, reflecting the growing trust in our platform as a solution for managing increasingly complex trials. This milestone underscores our strengthened market presence and the growing demand for centralized, compliant trial oversight. Fueled by a 254% increase in active studies, for 320% more unique users, our platform has become the preferred choice for clinical trial coordination. This exponential growth not only reflects user trust but also strengthens the collaborative power of our growing network.
As digitalization of trial processes accelerates, Cloud Concinnity continues to lead the way by centralizing documentation and processes, eliminating the use of disparate point solutions that are inefficient and increases the risk of data leaks/compliance issues.
2. Top Pharma Tech Software Provider Recognition
For the second year in a row, Cloud Concinnity was recognized by multiple industry leaders as a Top E-Clinical Trial Management Solution, further solidifying our standing as an industry leader and a trusted partner in clinical trial management.
Ensuring seamless coordination among multiple stakeholders is complex and resource-intensive. Cloud Concinnity enables a unified process of conducting independent safety review, avoiding cumbersome and time consuming document collaboration with emails and/or various document repositories.
3. Partnering with Industry Leaders to Improve the Platform
User feedback has been central to our success in continuously expanding and improving the platform. In 2024, we delivered quarterly software updates, enhancing functionality, performance, and user experience. These updates ensure our platform remains scalable, meeting the demands of a rapidly evolving clinical trial landscape.
4. Sustaining Part 11 Compliance
Compliance is integral to our mission, so maintaining Cloud Concinnity as a validated Part 11 compliance platform is important to us. In 2024 we completed rigorous audits, both internal and external, demonstrating our meeting of those requirements.Every software update adheres to exacting regulatory standards, ensuring trust and reliability for our clients.
Regulatory/compliance teams and study requirements are unique and must follow FDA and Charter specs. For organizations to be ever audit-ready, Cloud Concinnity provides a secure, centralized, and compliant foundation for reporting.
5. Expansion of Academic Medical Center Solutioning
This year marked a significant milestone as we partnered with a leading academic medical center research institution. Through this collaboration, Cloud Concinnity demonstrated its versatility across multiple use cases, showcasing its potential to revolutionize clinical research within academia. As we expand our academic partnerships, early adopters will play a pivotal role in shaping the future of trial coordination and gain exclusive access to tailored implementation support.
6. Strategic Team Growth
To support our expanding client base and the growing recognition of the imperative to digitalize trial processes, we grew our team this year with new industry expertise in functional areas and on our Clinical Trial Advisory Council, ensuring delivery of the best software and service for organizations transitioning to technology supported clinical trial oversight.
A Vision for 2025 and Beyond
As we reflect on the progress made in 2024, our focus now shifts to the future. With every partnership and every update, we are shaping a world where centralization of processes, collaboration across stakeholders, and compliance with regulations drive better outcomes for clinical trials.
By continuing to harness process intelligence and fostering innovation, we aim to empower our clients to stay ahead in an ever-changing industry. Together, let’s build a smarter, more efficient future for clinical trial coordination.
Here’s to an exciting 2025!
The Concinnity Company Announces latest release of Cloud Concinnity, its award winning eClinical Trial Management Software
NASHVILLE – November 18, 2024
The Concinnity Company has announced the release of Cloud ConcinnityⓇ 3.3.0. Cloud Concinnity is an integrated process management platform for clinical trial oversight. Concinnity was recently named a Top Ten eClinical Trial Management Solution Provider.
Clinical trial sponsors and clinical research organizations (CROs) manage dozens of ongoing clinical studies simultaneously throughout the year. Each of these studies requires centralization of data to create a single source of truth, and to both facilitate and control access. Vast teams of people collaborate across geography and time zone requiring the ability to work asynchronously on information and also meet as required by FDA and study protocols. Cloud Concinnity has become the go-to solution for centralization, collaboration and compliance challenges for hundreds of users managing processes crucial to improving global health and well being. Their usage of our platform and commitment to their work means Cloud Concinnity gets better and better with every release.
Cloud Concininty’s 3.3.0 release further enhances our meeting scheduling functionality. It is designed specifically for scheduling meetings among sponsors, committee members and other required parties. Selection of available times are now even easier to select from calendar views. And we’ve solved a time zone issue for teams with users in several different time zones.
The workforce challenges in the industry, availability and turnover, challenge security, efficiency and training. Cloud Concinnity has enhanced features to facilitate staffing changes, both temporary and part time, that ensure ongoing consistency, efficiency of compliances of required processes and services.
Additional enhancements include our system generated reminders features and changes to two factor authentication across challenging geographies.
Concinnity is 21 CFR Part 11 compliant platform is architecting the paradigm of tech-driven clinical trial oversight. The ecosystem has grappled with inefficiency and outdated technology for far too long. Current solutions rely on manual efforts and multiple tools. This approach is simply unsustainable.
Cloud Concinnity brings centralization, collaboration and compliance to mission critical clinical trial processes. The result? Process standardization & optimization and enhanced capacity & controls that drive efficiency, growth, and preventive compliance. Cloud Concinnity’s powerful, integrated workflow engine ensures better outcomes at lower cost.
To Learn More about Cloud Concinnity Contact:
[email protected]
(615) 988-1501
About Concinnity:
Cloud Concinnity® is an integrated software platform for clinical trial oversight. As a single, secure platform to manage, execute and record key processes, it drives a higher level of efficiency, speed and risk mitigation. Cloud Concinnity also unlocks the value of big data buried within multiple systems. The result is better product lifecycle management and decision-making. Concinnity transforms information flows, communications, and processes. By standardizing and automating these activities, treatments can reach patients faster and at a lower cost. We are dedicated to harnessing the power of process, innovation, and technology to create a stronger, healthier world. For more information on how we are building a better future one clinical trial at a time visit The Concinnity Company.com
Bridging Expertise: How Sponsor-CRO Collaboration is Shaping the Future of Clinical Trials
Ever wonder what fuels the success of a clinical trial? It boils down to collaboration—bringing together diverse teams of sponsors and Contract Research Organizations (CROs), often spanning multiple geographies. Sponsors take the lead by setting the course, securing funding, and ensuring that every regulatory requirement is met. They are responsible for designing the trial protocol, selecting sites, and making critical decisions to keep the trial on track.
On the other hand, CROs offer specialized expertise in managing the intricate processes involved in executing a trial. These teams include project managers, research assistants, site contract managers, quality managers, clinical research associates, clinical trial assistants, regulatory affairs managers, heads of clinical operations, data managers, statistical programmers, and biostatisticians. Each of these roles is vital in ensuring a successful trial outcome.
Rising Complexity and Shifting Models in Clinical Trials
As the pharmaceutical industry continues to evolve, clinical trials are becoming increasingly complex. The globalization of R&D has introduced new challenges, with 51% of drug developers citing trial complexity as a significant hurdle and 46% identifying regulatory demands as a close second, according to a survey by Thermo Fisher Scientific. To manage these growing demands, pharmaceutical companies have increasingly turned to Contract Research Organizations (CROs), with nearly three out of four clinical trials now managed by these organizations.
Traditionally CROs have offered full-service outsourcing (FSO) models to pharmaceutical and biotechnology companies to support clinical trial operations. However, a significant shift toward functional service provider (FSP) models has been gaining traction. This shift is not just a trend; it’s becoming the new norm. The top 10 pharmaceutical companies have adopted FSP models, and nearly half of the companies ranked 11th to 20th are using a combination of FSP and FSO models. This change is being driven by several market forces, including a focus on bolt-on M&A strategies, the need to reduce costs, improve operational efficiency, decrease cycle times, provide specialized services, and maintain more strategic control over clinical trials.
Technology: The Key to Improved Clinical Trial Coordination
As the FSP model becomes more widespread, both sponsors and CROs are increasingly relying on technology to streamline clinical trial operations. While CROs traditionally made most technology-related decisions, now sponsors are also taking a more active role in selecting and leveraging technological solutions. This shift towards integrated platforms is crucial for fostering collaboration and ensuring efficient trial coordination.
This is where cloud-based platforms like Cloud ConcinnityⓇ play a crucial role. By streamlining workflows and enhancing communication, these platforms enable more efficient collaboration between sponsors and CROs. Cloud technology facilitates smoother interactions, aligning all stakeholders and driving better outcomes throughout the trial process.
Explore our blog to learn how cloud-based platforms and automation are revolutionizing clinical trial coordination.
A Case in Point: Tackling Inefficient Clinical Trial Collaboration
To highlight the impact of collaboration and technology, consider the challenge faced by one of our customers: inefficient document management and communication delays. Their trial leaders struggled with a high volume of emails and multiple document versions, making it difficult to track changes and maintain version control across platforms. This inefficiency significantly impacted their overall trial timelines.
The Solution: Streamlined Collaboration and Document Management
We provided a real time collaboration platform that addressed these challenges by enabling better communication and simplifying document management. The platform’s key features included:
Centralized Document Repository: All documents were consolidated into a single, secure location, eliminating the need to manage multiple versions across different platforms
Real-Time Collaboration: Stakeholders could now collaborate on documents simultaneously, ensuring real-time updates and reducing confusion caused by conflicting versions
Version Control: Automatic version tracking ensured that the most recent document was always accessible, minimizing errors due to outdated information
Role-Based Access: With secure, role-based permissions, only authorized users could view or modify documents, improving document integrity and confidentiality
The Impact: Improved Efficiency and Accuracy
The results were immediate. With less time spent on document management and email exchanges, teams were able to focus on more critical tasks. The platform's role-based access and integrated signature requests sped up the approval process, ensuring that only authorized personnel could review and approve documents swiftly.
Moreover, by reducing clerical errors and ensuring clear audit trails, the platform simplified documentation processes. This created more accurate records and a reliable, efficient audit process.
Closing Thoughts
By fostering collaboration among diverse opinion leaders, our platform played a pivotal role in transforming the clinical trial management system. Not only did it improve coordination, but it also accelerated trial timelines, ensuring that different perspectives were integrated seamlessly into the process.
The importance of collaborative trial management cannot be overstated—especially in today’s increasingly complex and competitive landscape. As clinical trials become more intricate, fostering collaboration across various experts is essential for achieving breakthroughs and maintaining a competitive edge. Technology, like Cloud Concinnity, enables this collaboration, making clinical trials more efficient, responsive, and aligned with the industry's evolving needs.
In the world of clinical trials, effective collaboration isn’t just a nice-to-have—it’s a must for success.
Improving Safety Reviews in Clinical Trials Through Tech-Enabled Centralization
Imagine having all your data seamlessly integrated in one place! A recent Oracle survey revealed that 50% of clinical data managers rely on up to five different data sources, making it challenging to draw meaningful insights from trial data. The complexity of managing multiple platforms creates data silos, isolating information and making it difficult for data managers to achieve a comprehensive view. This fragmentation not only increases the risk of data inconsistencies but also slows down the decision-making process, hampering the overall efficiency of clinical trials.
Beyond data consolidation challenges, the use of disparate systems leads to broader operational complications. Disparate solutions designed for specific tasks are often not scalable or adaptable to new technologies, requiring costly modifications to remain compliant with evolving regulations. Additionally, managing communication through unsecured methods like email increases the risk of data privacy issues—a critical concern in today’s digital transformation in healthcare, and corporate cross-contamination. This fragmented user experience undermines operational quality and increases inefficiencies, as more time spent on management and training drives up costs and reduces trial management effectiveness. However, with Cloud Concinnity®, costs could potentially be reduced, leading to a 30-50% ROI and enhancing overall efficiency.
The pharmaceutical industry is moving away from traditional point systems—discrete tools designed for individual tasks—towards centralized cloud platforms. These unified platforms centralize all trial-related data, eliminating inefficiencies and improving data integrity. This shift represents not just a technological upgrade but a fundamental rethinking of how trials are managed, offering the potential to enhance overall research quality.
Cloud Concinnity® is at the forefront of this transformation, serving as a centralization hub for safety review activities. By offering streamlined access to essential tools from any location, Cloud Concinnity effectively resolves the complexities of managing multiple disparate systems. Its key features include:
Automated Task Alerts: Keeps teams aligned with timely reminders, ensuring tasks are completed efficiently and on schedule
Time-Saving Safety Listings: Streamlines safety data review, reducing manual effort and speeding up the process
Document Distribution via Standard Workflows: Automates the distribution process with consistent workflows, minimizing administrative workload
Role-Based Access Control: Ensures secure access control and seamless user transitions while minimizing data breach risks. Cloud Concinnity also includes a 21 CFR Part 11 compliant login with 2-factor authentication for enhanced data security
Robust Notification Center: Sends automated reminders to keep teams informed, enhancing coordination across dispersed teams
Centralized Dashboard: Provides a real-time overview of all studies and processes
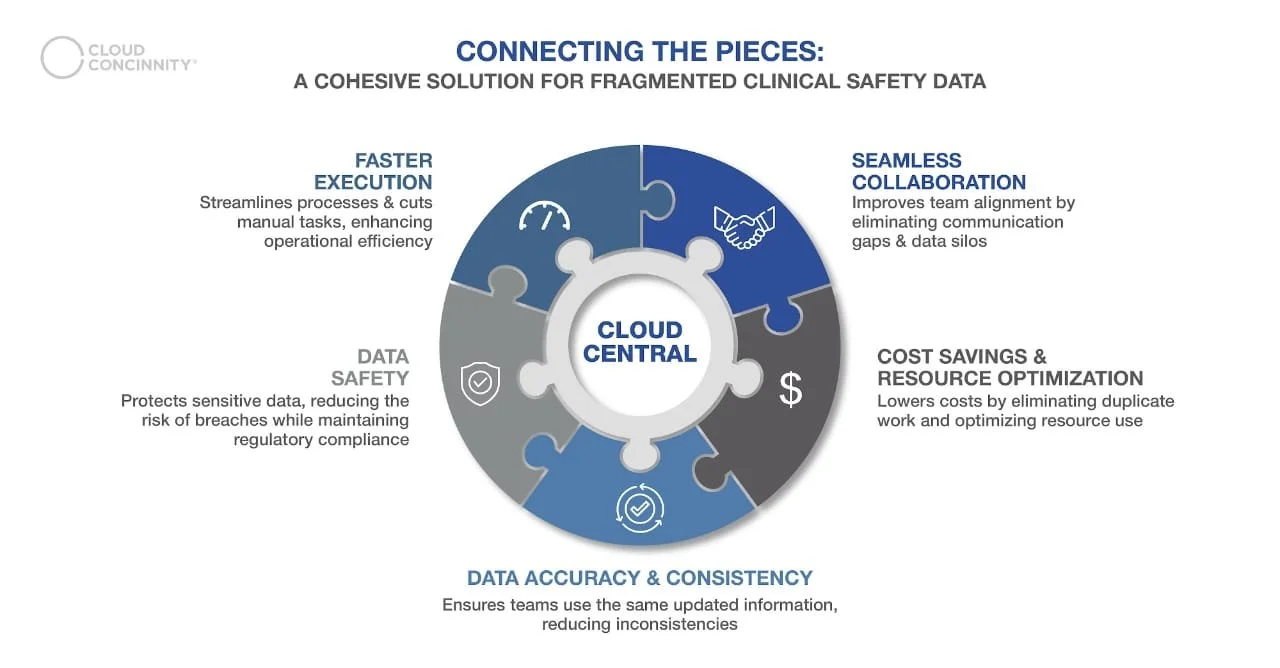
Cloud Concinnity’s centralizing features offer several overarching benefits, like.
Seamless Collaboration: Teams across different locations can collaborate in real time with unified access to up-to-date data, speeding up decision-making and fostering greater coordination
Data Accuracy and Consistency: Centralized management eliminates redundancies and ensures data integrity, so every site operates on accurate, updated information without duplication or confusion
Data Safety: By mitigating breach risks and maintaining regulatory compliance, Cloud Concinnity safeguards sensitive information, ensuring secure data handling throughout the trial process
Faster Execution: With automated alerts and faster safety reviews, Cloud Concinnity reduces the time spent on manual tasks, accelerating the clinical trial process
Cost Savings and Resource Optimization: By reducing administrative burdens and avoiding duplicative work, Cloud Concinnity helps streamline operations, ensuring that trials remain on budget while improving overall outcomes
There has always been a clear need for centralized platforms in clinical safety review processes, but sponsors were hesitant to adopt them due to various factors, particularly data privacy concerns. Impact of COVID-19, however, drastically changed this perspective. With social distancing and remote work becoming widespread, the limitations of fragmented systems became apparent. Centralized platforms quickly emerged as the more reliable and efficient solution, offering streamlined data handling, and a cost-effective approach. As a result, leveraging centralized technologies has become critical for the future success of clinical trials.
Looking ahead, the future of clinical research will be increasingly shaped by the integration of advanced technologies, with AI in Healthcare and machine learning playing a central role. These advanced technologies will enable automated data quality checks, statistical analysis, and visualization techniques, rapidly identifying patterns, inconsistencies, and missing data. Centralized platforms, powered by AI and automation, will play a pivotal role in making clinical trials faster, more reliable, and more secure, ensuring that research adapts to the growing complexities of the healthcare landscape.
Transforming Clinical Trials’ Coordination Through Automation
Discover how automation in clinical trials tackles delays and inefficiencies by streamlining data management, enhancing collaboration, and ensuring regulatory compliance for independent safety review committees. Learn how this shift optimizes resource use, accelerates time-to-market, and supports more reliable trial outcomes
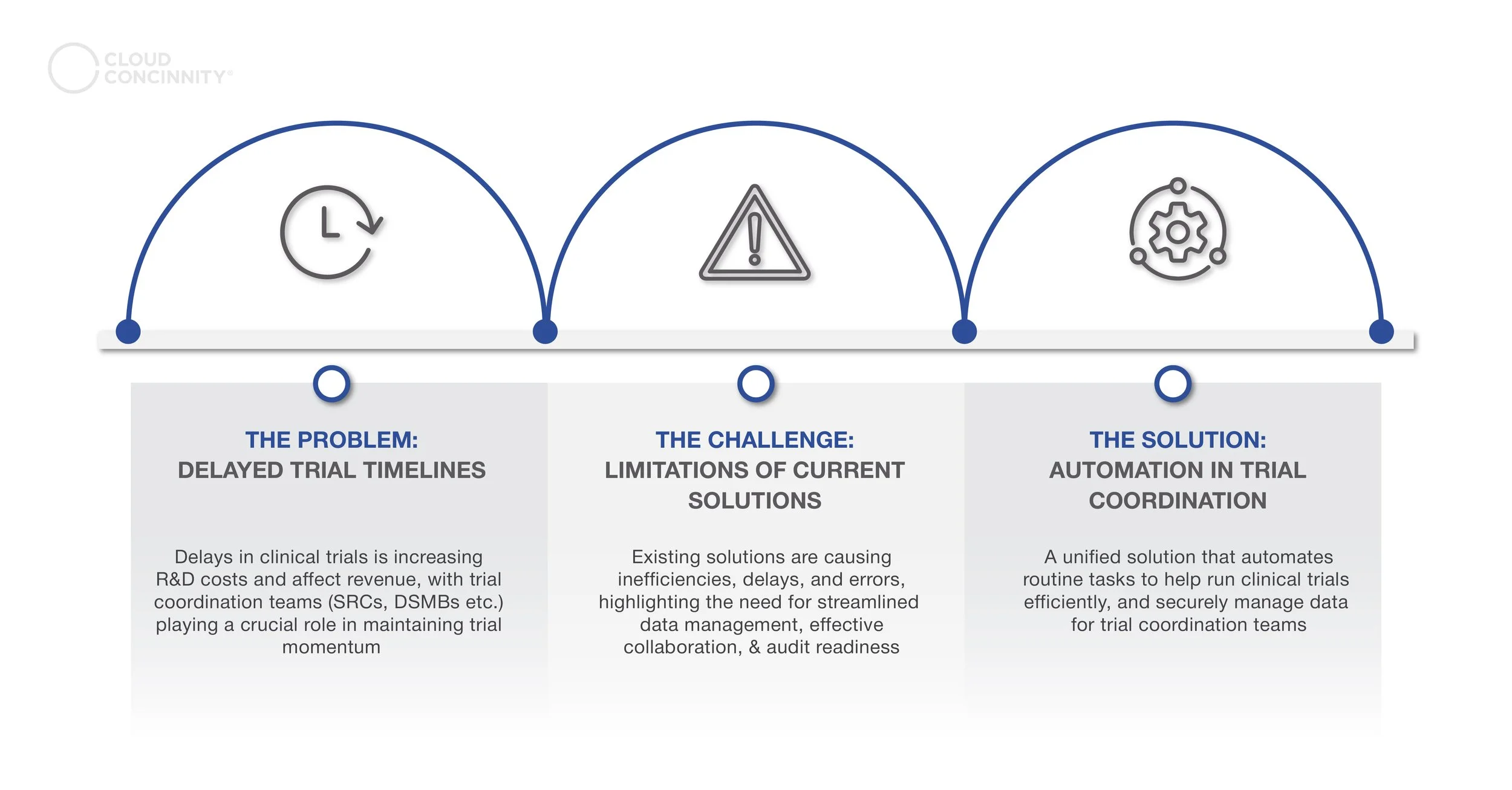
Clinical trial delays are a common challenge in drug research and development. Eight out of ten trials experience setbacks, with around 94% of these delays lasting longer than a month. This often results in increased costs, reduced profitability, or, in some cases, project failure. The root causes of these delays are frequently linked to unmet or complex regulatory compliance standards, gaps in procedure management, inefficient processes, and communication breakdowns between teams.
While it’s impossible to account for all unexpected challenges in today’s complex research environment, there are effective strategies to minimize the likelihood of clinical trial delays. One key approach is the integration of automation into clinical trial management.
Clinical Trial Coordination Committees play a vital role in ensuring patient safety and the scientific validity of trials. However, the current trial coordination landscape is complex, involving numerous stakeholders, intricate processes, and large volumes of data, all of which can negatively impact efficiency and contribute to delays.
Transforming Clinical Trials’ Coordination Through Automation - Problem , Challenge and Solution
The rise of automation technologies is reshaping clinical trial coordination. Traditional trial processes, which involve complex workflows managed by multiple teams, often lead to inefficiencies, delays, and errors. Automation simplifies these workflows by reducing repetitive tasks and centralizing operations, making trial management smoother and more streamlined. This shift not only enhances data quality but also helps detect unexpected variability that could affect trial outcomes. As a result, automation optimizes resource use, accelerates time-to-market, and alleviates staff workloads, ultimately making the clinical trial process more efficient and reliable. Many of the daily challenges faced by teams, such as independent safety review committees, can be alleviated with an automated solution. Here are the top five challenges experienced by clinical trial coordination committees:
1. Centralized Data Management: Clinical Trial Coordination Committees must carefully manage both blinded and unblinded data to avoid mistakes. Managing multiple committees across different trials, and accessing safety data without a unified platform increases the risk of data leaks and compliance issues, which can lead to inefficiencies and delays. Centralization of this process will help streamline both data management and trial coordination.
2. Document Management and Collaboration: Producing, sharing, and storing standardized documents like charters, agendas, and minutes can be challenging. Without a centralized system, documents become scattered, causing inconsistencies, while relying on emails or traditional repositories makes collaboration cumbersome and time-consuming.
3. Audit Readiness and Compliance: Achieving audit readiness and maintaining compliance requires detailed documentation and frequent updates. Regulatory teams must adhere to specific FDA and Charter requirements for each study. Clear eTMF documentation of Safety Listings, Document Distribution, and documented decision-making (eg. recommendation letters), is also essential to ensure all processes are accurately recorded and readily accessible for audits, a task that is challenging without the right platform.
4. Communication and Task Management: Organizing Trial Coordination Committees’ meetings across time zones is challenging and time-consuming without centralized scheduling tools, leading to delays and poor communication. Coordinating with statisticians for reports and sharing committees’ decisions throughout a trial requires strict procedures, and without the right tools, there's a real risk of things getting off track.
Addressing these challenges requires a decisive move toward automation. Automating routine tasks helps committees manage information more securely and run trials more efficiently. Cloud Concinnity has identified three key areas where automation can enhance the efficiency of clinical trial coordination:
1. Centralization: Cloud Concinnity’s Global Dashboard consolidates all projects into one hub, eliminating the need for multiple platforms. It provides secure, role-based access, centralizes key documents, and offers automated task alerts. Efficient safety listings and document distribution save time, while streamlined account management simplifies platform access.
2. Collaboration: Cloud Concinnity enhances collaboration with real-time document editing for faster revisions and accelerated approvals. It ensures secure teamwork through controlled access, automates access transfers during personnel changes, and integrates with Adobe Sign & DocuSign for easy, compliant signing.
3. Compliance: Cloud Concinnity ensures audit readiness with its Regulatory-Ready eTMF Integration. It streamlines sponsor audits with efficient access controls and customizable workflows to meet FDA requirements, while visualized tracking keeps committees on top of regulatory needs.
Automation has become a necessity in healthcare, especially in clinical trials where speed and safety are paramount. By streamlining processes, automation overcomes the inefficiencies of traditional methods, driving operational success.
To effectively incorporate automation in clinical trials, it's crucial to identify the right tasks for digital transformation. Processes such as document management, signal detection, and safety reviews are ideal for automation, as they are data-heavy and involve repetitive work. This approach not only improves efficiency but also enhances customer benefits and addresses specific operational problems, ultimately driving better trial outcomes and regulatory compliance. Effective automation requires not only the right tools but also staff education. Training should enhance digital literacy, helping teams identify automation opportunities and focus on higher-value work.
Automation is not just transforming clinical trials—it's reshaping the future of patient safety and operational excellence, ensuring that life-saving treatments reach the market faster, safer, and more efficiently than ever before.
Learn more about Cloud Concinnity® and schedule a demo today.
Risk Mitigation in Clinical Trials: How Cloud Concinnity® Helps Organizations Minimize Risk
Cloud Concinnity® provides invaluable help for clinical trials administrators by centralizing information securely in the cloud, automating critical compliance processes and pushing critical evaluations and alerts to decision makers.
As clinical trials progress and the risk of potential issues within the process rises, organizations are tasked with finding a way to efficiently manage these risks without impacting costs or pushing back schedules. Managers tasked with clinical trial oversight are at the epicenter of analyzing and controlling these evolving risks.Cloud Concinnity® provides invaluable help for clinical trials administrators by centralizing all information securely in the cloud, automating critical compliance processes and pushing critical evaluations and alerts to decision makers.
Cloud Concinnity® equips teams with automated alerts, assessments, collaboration tools, and more, giving them complete control over their data and mitigating risk while helping to ensure they remain compliant.
How Cloud Concinnity® Helps Minimize Risks in Clinical Trials
Clinical trials are essential in ensuring that new treatments and drugs are safe and effective for patients. However, they come with a certain level of risk that can be difficult to manage. That's where Cloud Concinnity® comes in.
From tracking participant information to securely storing trial data, Cloud Concinnity® offers a comprehensive solution to help trial sponsors and their administrators focus on what matters: ensuring patient safety, treatment efficacy and regulatory compliance. With its user-friendly interface and advanced security features, Cloud Concinnity® is poised to transform the way clinical trials are conducted.
Some of our client's favorite risk-management features include:
Centralized Information Management
Workflow Automation
Centralized Meeting Management and Collaboration
Audit-Ready Information Output
Verified Security and Regulatory Compliance
1. Centralized Information Management
With the ongoing digitization of clinical trials, how study data is captured and recorded is undergoing significant changes. This transformation, in turn, has a substantial effect on the way oversight committees assess data. Our cloud-based platform serves as a centralized hub for the clinical trial information governance and oversight ecosystem - streamlining access to necessary tools and making them available from any location through significant web browsers.
Our easy-to-use interface is built to allow study administrators to grant or revoke system access to specific individuals based on their roles. Thus, users can only access the information they need to complete their work.
Role-based access is paired with a robust notification center to inform your team with reminders that can be sent through text and email.
2. Workflow Automation
Cloud Concinnity® uses automated workflows to provide a double-whammy of risk mitigation. First, you can ensure critical tasks and communications are sent out without issue or manual involvement by automating your mission-critical workflows. This helps to free up additional time, allowing your team to focus on other tasks - reducing labor overhead and streamlining operations.
Second, automating repetitive workflows ensures process consistency, which is critical in clinical trials to ensure patient safety and regulatory compliance. Process quality is difficult to control when manual and almost impossible to prove in terms of compliance. Cloud Concinnity virtually eliminates this risk.
3. Centralized Meeting Management and Collaboration
Who hasn't wasted hours trying to schedule a meeting with multiple team members? Cloud Concinnity® is here to help with end to end meeting management
Our robust platform comes equipped with a host of features to help you schedule meetings, including:
Online Meeting Polling Functionalities
Automated Meeting Reminders
Video Conference Integrations
Calendar Syncing
All of these work together to save administrators time while allowing committees (like DMCs) to stay up-to-date with critical updates and mitigate any risk of meetings NOT happening according to protocols, critical timeframes and regulatory requirements.
4. Audit-Ready Information Output
Cloud Concinnity® was built to meet the specific reporting needs of Trial Sponsors CROs and their oversight teams. As such, our platform has push-of-a-button access to information required for external and internal audits.
This functionality includes audit-ready tracking of tasks, messages, role changes, meeting minutes, documents, and critical actions taken and more.! Your team can use the standard or custom eTMF and FDA templates to create audit and compliance documents.
5. Verified Security and Regulatory Compliance
Tools and processes with verified security and regulatory compliance are crucial for clinical trial risk management because it ensures that your organization operates and complies with applicable laws. This compliance also helps to demonstrate the trial organizer's commitment to ensuring the safety and security of its participants, employees, and stakeholders.
Cloud Concinnity® has a host of security and compliance features, including:
Two-Factor Authentication
SOC 2 Attestation
21 CFR Part 11, Annex 11, GDPR and HIPAA Compliance
Risk mitigation in clinical trials is essential for ensuring patient safety and process efficacy. Cloud Concinnity® helps organizations minimize risks with a suite of features built specifically for clinical trial oversight. Learn more about Cloud Concinnity® and schedule a demo today.
Trial Success Hinges on Human Capital: How to Recruit Top Clinical Trial Managers
Here are some of the best practices for recruiting quality clinical trial managers, who can elevate a clinical trial and help to ensure its success.
Clinical trial managers play a critical role in ensuring a trial's success. As you know, every decision and action taken by the manager impacts its originator's goal. The best clinical trial managers can elevate a clinical trial and ensure success.
What are the best practices for recruiting quality clinical trial managers? Read more to uncover tried-and-true methodologies that will improve your chances of successfully bringing in top talent essential to your next endeavor.
The Importance of Clinical Trial Managers and the Need for Top Talent
In clinical trials, recruiting the right team is critical – from pre-selection through onboarding – as this human capital lays the foundation for trial progression. Clinical trials need people with intelligence who can adapt to the evolving world of clinical trials.
With a strong team in place, clinical trial timelines can be completed, data quality can be maintained, and costs can be mitigated. Given the current emphasis on innovative treatments and new drugs, the demand for top talent in this field is at an all-time high.
Trial Success Hinges on Human Capital: How to Differentiate Your Organization and Recruit Top Clinical Trial Manager
Diversify Your Recruitment Channels
Not all recruitment channels are created equal. Recruiting has come a long way from job postings and word-of-mouth referrals. While those methods still have their place in the modern job market, traditional recruiting tools have evolved to include online job boards, social media platforms, and applicant tracking systems.
Companies must diversify their recruitment channels and leverage technology to reach a larger pool of qualified candidates. By utilizing modern recruitment tools, employers can reduce their reliance on conventional methods and expand their ability to connect with potential applicants.
Refine Your Strategies For Evaluating the Right Clinical Trial Manager
Once you start attracting talent, it's time to begin evaluating your prospects so that you can find the ideal clinical trial manager. We recommend using an objective system of evaluation. Your organization needs to have a process that allows it to review the skills, knowledge, and requirements for a particular role and then use technology such as applicant tracking systems to evaluate candidates on their merits.
Additionally, it's essential to analyze the soft skills of your clinical trial manager. Communication, multitasking, and people skills are all critical elements of a successful clinical trial manager. As such, it's recommended to conduct multiple interviews where you and your team can ask questions about potential candidates' practical experience and soft skills.
Provide an Improved Working Quality of Life Through Superior Technology
In today's hiring market, salary and benefits are NOT the only determining factors for landing top-tier talent. Companies must look beyond these enticements and recognize the value of improving working quality of life. One of the most straightforward methods to meet this objective is through superior technology.
Our recommendation: Invest in a clinical trial oversight management tool that is built to meet the specific needs of clinical trials - Cloud Concinnity®.
Cloud Concinnity® provides a host of features that improve the quality of life for your entire organization, including your clinical trial managers. These features include:
Workflow automation to manage repetitive tasks.
Meeting scheduling tools to make it easier to coordinate multiple people's schedules.
Videoconference Integrationsfor streamlined meetings video conferencing.
A centralized cloud-based library to house critical documents, data points, patient information, and meeting notes.
And more!
Interested? Learn more about Cloud Concinnity® with a hands-on demonstration.
Benefits of Investing in Human Capital for Clinical Trial Success
Proper management of clinical trials is both complex and costly. By investing in human capital, CROs can set themselves up for success in the competitive and ever-evolving field of clinical trials. Tools like Cloud Concinnity® can help improve your trial managers' quality of life and give your team an advantage in sourcing and recruiting new talent.
No Going Back: 10 Trends That Have Changed the Life Sciences Industry Forever
Life sciences industry trends point to a world with fewer barriers, faster drug development, and more efficient communication. Here are ten things life science professionals need to know to stay ahead in 2023.
The life sciences industry has undergone seismic shifts in the three years, with sweeping changes across research, development, and commercialization. Industry trends point to a world with fewer barriers, faster drug development, and more efficient communication. Here are ten trends life science professionals need to know to stay ahead in 2023.
10 Changing Life Sciences Industry Trends
1. Focusing on Health Equity:
Equality and access to healthcare are becoming increasingly essential policy concerns. Companies are investing in projects that provide equitable health care, such as remote monitoring devices and telehealth technology, to reach populations who have not historically had good access to healthcare.
2. Big Data:
The shift from a fee-for-service reimbursement model to one based on value, data, and analytics will become even more critical. Big data has the potential to provide insights into medical trends and treatments that were not previously available, which can help improve outcomes for patients and reduce costs.
This shift towards value-based reimbursement models is creating a new emphasis on using analytics and other data-driven approaches to healthcare. As health systems increasingly rely on predictive models and algorithms to inform decisions, ensuring that these models are appropriately validated and produce reliable results will be critical. Additionally, this data can be used to create personalized treatments for patients that optimize outcomes while reducing costs.
3. Personalized Medicine:
With new technology comes an evolution in personalized medicine. Advances in genetics and genomics are enabling a shift towards a more personalized approach to healthcare, with treatments tailored to individuals. This is helping to improve outcomes for patients and reduce costs for providers.
4. Accelerating Digitization:
COVID-19 exacerbated the digital transformation in every healthcare industry, forcing sectors to adapt to the no-contact world. Think of the vast strides just in the past two years. A considerable increase in telehealth (remote healthcare visits, decentralized clinical studies, etc.) and advanced medical devices (wearables and sensors) is just a small part of modern medicine.
5. Rise of AI and ML:
Advances in artificial intelligence (AI) and machine learning (ML) are revolutionizing the life sciences industry. AI can quickly analyze sizeable amounts of data, enabling companies to develop drugs faster and cheaper.
6. Scaling Cloud Platforms and Securing Data:
Clinical trial managers increasingly rely on cloud-based platforms to store and manage data associated with clinical trials. As the amount of data being collected continues to increase, it is critical that clinical trial managers have access to secure and reliable cloud platforms that can effectively scale up as needed.
Additionally, they must be proactive in implementing stringent security protocols to protect patient data and ensure compliance with HIPAA and GDPR. By properly managing their cloud infrastructure, clinical trial managers can ensure data safety while ensuring operational efficiency.
7.. Shortening Vaccine Development Timeline:
The pandemic accelerated the development, testing, and distribution of drugs and vaccines more than ever. Official regulators like the FDA and EMA played a key role in our speedy progress over the last few years, and it won’t slow down.
Infrastructure has been developed over the years to keep pace and never slow down, and the trends show that these swift projects are going to be the norm going forward.
8. Increasing Reliance on Data Analytics
With an increasing number of clinical trials being conducted, the ability to quickly analyze large datasets is becoming more important to pharmaceutical companies. Data analytics tools are helping researchers understand the impact a potential therapy or drug has on a patient population much faster than ever before.
9. Adoption of Biomarkers
Biomarkers are becoming an increasingly important tool for evaluating patients in clinical trials, with researchers able to measure more precisely how a potential therapy or drug impacts an individual.
Clinical trial managers must be prepared to embrace the use of biomarkers in their clinical trials. Biomarkers provide valuable insights into disease processes, allowing for more precise diagnosis and treatment. By leveraging these types of molecular signatures, clinical trial managers can quickly and accurately detect changes in a patient’s condition, which could indicate response to treatment. Additionally, biomarkers can help clinicians stratify patients more precisely, providing more accurate information on who is most likely to respond favorably to specific treatments.
For clinical trial managers to effectively use biomarkers in their studies, they need the right infrastructure and data management solutions in place. Sufficient storage should be available so that all biomarker data can be collected and stored safely and securely. This data should also be structured so that it can easily be analyzed with AI or ML algorithms. By ensuring that their technology stack is equipped to handle the demands of a biomarker-based study, clinical trial managers can ensure that they are able to maximize the potential benefits of this powerful tool.
10. An Increase in Industry Mergers and Acquisitions
The healthcare industry has been trending towards more mergers and acquisitions for the past few years. “...the 10 largest biopharma M&A deals in 2022 totaled around $65 billion. While that was an increase from $53 billion in 2021, it was a notable downturn from prior years.”
Tens of billions of dollars are moved around each year for these massive companies to collaborate and create better medicine. With these large conglomerates in place, more medicine can be generated, more clinical trials will be conducted, and real medical breakthroughs can become possible.
From the rapid adoption of digital technologies to the emergence of new therapies, the life sciences industry has seen dramatic changes over the last decade. This continual evolution has created an environment that is more dynamic and nuanced than ever before, with opportunities and challenges presenting themselves every day.
With these changing trends in mind, it’s clear that there’s no going back to how things were before – and this is a good thing. Life sciences companies must be prepared to embrace new approaches while staying rooted in their mission of improving health outcomes worldwide. We can look forward to a brighter future with continued innovation and determination.
Invest in Cloud Concinnity to Improve The Clinical Trial Experience
The Cloud Concinnity platform was built to meet the specific needs of clinical trial managers, CROs, and DSMBs. Our cloud-based system provides trial stakeholders with a centralized platform for patient information, communications, processes, and reporting onto a single, secure platformhub to improve their quality of life.
To learn more, schedule your demo today.
8 Technology Trends That Are Impacting the Clinical Trial Space
Technology’s impact on clinical trial management touches patient recruitment and retention, study design and execution, and compliance and reporting. Here are 8 technology trends affecting the clinical trial space.
Technology’s impact on how clinical trials are managed is profoundly changing how treatments move from research and development to market. This seismic change impacts everything from patient recruitment and retention to study design and execution, as well as affecting compliance and reporting results. Understanding how these trends shape the trial management process can help you successfully navigate this changing landscape.
Here are eight technology trends changing the pharmaceutical - and thus clinical trial - space.
8 Tech Trends Impacting Clinical Trial Management
Data Accessibility
One critically important trend that’s coming into focus for drug makers is that patients are increasingly taking control of their healthcare decisions and expectations around treatment options. As a result, they now have more access to information than ever before – including data on both existing approved therapies and experimental drugs through online patient support groups, online media, and social networks.
This access to information has empowered patients to be more informed about the different options available to them, which in turn helps drive greater involvement in their clinical care decisions and trial involvement.
Artificial Intelligence
Another key trend driving change in the clinical trial space is the increased use of artificial intelligence (AI). Traditionally, AI was used primarily for data analysis and machine learning. Today it’s being applied across various aspects of drug development – from reducing costs, improving patient outcomes, enhancing early-stage research and streamlining trial processes.
For example, using AI to predict issues or potential adverse events during clinical trials can help reduce development timelines while also improving patient safety.
Virtual and Augmented Reality
In addition to AI, other emerging technologies are having a major impact on clinical trials – namely, virtual and augmented reality (VR/AR). These immersive technologies have the potential to revolutionize many aspects of the drug development process – from patient recruitment and engagement to improving study design. For example, VR may help enhance patient comprehension and retention by immersing participants in simulated environments that mimic trial processes. On the other hand, AR could be used to enable remote monitoring of subjects during home-based trials.
Blockchain Technologies
Blockchain technologies are also poised to disrupt the pharmaceutical industry. The use of blockchain in clinical trials offers a number of advantages that can help increase both transparency and data security. As an example, by creating a distributed ledger for each trial participant’s clinical data, it would be possible to share this information with authorized parties – such as patients and researchers – while still maintaining confidentiality. In addition to these benefits, blockchain could also help pharmaceutical companies streamline clinical trial management processes and improve patient retention rates.
Cybersecurity
While each of these trends offers tremendous potential for improvements in clinical trials, it’s essential to keep in mind that they also bring new challenges and risks. As drug makers continue to embrace innovative technologies and processes, the need for proper data security has never been greater.
Ensuring that appropriate cybersecurity measures are in place will help avoid potentially devastating data breaches – such as those that have recently occurred at some major pharmaceutical companies.
Continuous Drug Production
Continuous drug production, also known as continuous manufacturing, is a newer methodology that enables the creation of pharmaceuticals in large quantities at any time. In contrast with traditional batch production methods that take weeks or months to complete, continuous processing allows manufacturers to produce drugs continuously and more quickly.
Due to their shorter development timelines and lower cost structures compared with legacy pharmaceutical companies, innovative biotechnology firms are taking charge of this initiative. However, even traditional drug makers are beginning to explore adopting this new tech.
This trend will lead to an increased number of generic drugs becoming available and further price pressure on branded medications already in the market. Additionally, as biopharma companies invest significantly in R&D for biosimilars (generic therapeutic proteins), the potential for these drugs to become available more quickly will also increase.
Telemedicine
The use of telemedicine in clinical trials has been steadily increasing throughout the last few years, and it shows no signs of slowing down anytime soon. With its ability to reduce clinical trial timelines by bringing together patients and physicians at remote locations, this tech trend is likely to become increasingly prevalent in pharma R&D.
As drug makers continue to refine their approaches using virtual physician consultations, they are expected to realize more cost savings while simultaneously improving patient outcomes. Moreover, as new regulatory rulings enable medical device and smartphone companies to directly market their products for clinical use, the potential for telemedicine to become an integral part of clinical trials will only continue to grow.
Big Data
In recent years, big data analytics have begun playing an increasingly important role in driving advancements in healthcare. As pharmaceutical companies leverage this technology to mine patient data and generate insights into disease progression, they may be able to predict drug efficacy and safety earlier in development more accurately.
Additionally, by using historical trial data along with real-world evidence from electronic health records (EHRs), researchers can gain valuable information on how patients managed their conditions outside of clinical research settings – and ultimately design more effective clinical trials. By embracing big data analytics at all stages of R&D, drug makers can gain valuable insights that may help them streamline the trial design and enrollment process and improve overall study execution.
Overall, there are several exciting tech trends currently reshaping the space While these trends present new challenges for clinical trial managers, they also provide an opportunity to deliver life-changing drugs and treatments more quickly than ever before. By proactively addressing these risks with appropriate technology measures in place, clinical trial managers can confidently navigate this changing landscape and successfully advance their trials through development and onto the market.
Get Ready for The Future of Clinical Trial Management with Cloud Concinnity
Cloud Concinnity was specifically built to meet the need for more efficient, effective clinical trial management. Our cloud-based integrated platform centralizes, simplifies, and automates control of data, communications, process management & reporting while unlocking the value of big data.
What does this mean for you?
Our clinical trial solutions enable sponsors and CROs to effectively manage large, complex trials. We allow you to reduce operational costs, eliminate data entry errors & increase the speed of decisions with one centralized platform that can be accessed anytime, anywhere. Our intuitive interface is designed to enable users to quickly understand their roles and responsibilities while taking advantage of automated processes.
The result, more efficient processing, a stronger ROI, and a better quality of life for your workforce.
Learn more about Cloud Concinnity and schedule your demonstration today.
7 Data Protection Tips for Data Safety and Monitoring Boards
To ensure the data being stored is secure and private, clinical trials must implement strong data protection measures to keep their clinical trial data secure. Here are 7 data protection tips for data safety and monitoring boards.
As data privacy becomes increasingly important in the clinical trial space, data protection, in turn, becomes a higher priority for data safety and monitoring boards. To ensure the data you are storing is secure and private, clinical trials must implement strong data protection measures to keep their clinical trial data secure. Here are 7 data protection tips for data safety and monitoring boards.
7 Ways Data Safety and Monitoring Boards Can Improve Data Protection
Reviewing and Establishing a Data Protection Policy for Your DSMB
Improving data protection starts by creating a data protection policy. Your data protection policy will provide answers and insights for all data protection questions and protocols.
This policy can be as robust or simple as your organization sees fit, but at a minimum, it should:
Outline the measures taken to protect data.
Outline the responsibilities of each party involved in the data collection process.
Include a list of data that should be protected.
Procedures for monitoring and responding to any potential security breaches.
Specify how long data will be retained and when it should be destroyed.
Train the Entire Team on Data Protection
Once you have your policy, it’s time to train the team. This will ensure that everyone knows the importance of data protection and knows the procedures for responding to any potential security incidents.
For training, choose the methodology that would work best for your clinical trial stakeholders - in-person, online, video on demand, workshops. The options are endless. Choose the one that works best and then ensure that the training covers all aspects of data protection, from encryption to audit and compliance procedures. The training should also cover their data protection responsibilities.
Regularly Monitor Data Access
Limiting and auditing data access is critical to strong data protection policies. DSMBs should routinely monitor data access to ensure that only authorized personnel have access to the data. This can be done through the use of audit logs, which track and document any data access attempts.
Third-party tools (like the Cloud Concinnity platform) can be used to provide DSMBs with a robust toolset to manage data access and monitor data compliance. To learn more about how Cloud Concinnity can help improve DSBMs data protection policies, schedule a demo here.
Utilize Firewalls and Network Security
A mix of firewalls and other network security measures can help DSMBs keep trial data safer. Firewalls can act as a barrier between your network and the outside world, preventing unauthorized access to your data. Network security measures such as intrusion detection systems and backup data solutions can provide additional layers of protection to keep data safe.
Use Encryptions Where Needed
Encryption is one of the most essential data protection measures. Encryption scrambles data so that it is unreadable to anyone without the encryption key. This ensures that even if the data is accessed by an unauthorized party, they won’t be able to interpret and use the data.
Start by encrypting any confidential trial or patient data that you store or process. You should also consider encrypting your company’s internal communications, such as emails and instant messages.
Implement Multi-Factor Authentication For Trial Stakeholders
Multi-factor authentication adds an extra layer of security to your systems. By requiring multiple pieces of information to access data, you can keep critical data safe in the event of a stolen password.
Several multi-factor authentication options are available, including mobile security codes, authenticator codes, or even biometric data points (such as fingerprints). The best choice for your trial is one that offers security without being too much of a nuisance for your team.
Perform Regular Security Audits
Regular security is an essential part of data protection. Regular security audits will allow your team to identify and address any potential security vulnerabilities before they become a problem.
On top of your systems, you should also audit your data protection policies and procedures to identify gaps and determine efficiencies. Remember, the policies are there to serve the clinical trial and the DSMB. If they aren’t working, don’t be afraid to make a change.
By implementing these data protection tips, data safety and monitoring boards can ensure that the data they are responsible for is secure and private. If your DSMB is searching for new methods to improve data protection, we can help. We built the Cloud Concinnity platform to help DSMBs protect their clinical trial data.
Pro Tip: With the Cloud Concinnity platform you can implement a workflow to automate the above - eliminating manual audits and ensuring security compliance. Learn more and schedule your demo of the Cloud Concinnity platform here.
5 Ways CROs can Improve Their Clinical Trial Manager Recruitment Efforts
An experienced clinical trial manager can be integral to the success of a CRO’s operations. Here are five ways that CROs can improve their clinical trial manager recruitment efforts.
As the clinical trial industry continues to expand in this post-COVID environment, Contract Research Organizations (CROs) need to hire more clinical trial managers to ensure the success of their studies. An experienced clinical trial manager can differentiate between a successful and unsuccessful trial. As such, they are integral to the success of a CRO’s operations. In this post, we’re sharing five ways that CROs can improve their clinical trial manager recruitment efforts.
Let’s get started.
5 Tips CROs Can Use To Attract More Clinical Trial Managers
1. Grow and Nurture Your Network
Industry events, conferences, and organizational socials allow CROs to expand their networks and meet potential candidates. Often recruiters may attend these events only if they have an urgent need, or they may attend but not make an effort to follow up with potential candidates only because they don’t have an immediate opening.
2. Offer Competitive Compensation and Benefits
If your CRO is searching for top-tier talent, it needs to ensure that it is providing a competitive compensation package. This includes offering both an attractive salary and additional benefits such as insurance, performance-based incentives, and signing bonuses.
Depending on your competitive market, you may need to offer additional perks, including flexible work options and other employee perks, retirement plans, and specialized training opportunities.
3. Enhance and Showcase Your CRO Brand
A positive work culture is also critical to attracting new talent. It’s not enough to have clear expectations and values. Your organization needs to embody these values across each team member - including new hires.
Start by creating a culture of respect for each other and a commitment to continued excellence and dedication to high-quality work. It’s also important to ensure that you provide an environment where employees feel valued for their contributions and are encouraged to express their ideas.
Need more convincing? A strong workplace culture of appreciation can also increase employee retention over the long term.
4. Optimize Your Hiring Process
Your hiring process is often the first interaction a potential recruit has with your organization. As such, it’s an opportunity to prove that you value and respect your employees while building trust with potential prospects.
Here are three steps you can take to optimize the clinical trial manager recruiting process:
Streamline the Application Process
Make sure that your application process is as straightforward as possible. This includes having a user-friendly online form for applicants to fill out and a clear description of the job requirements and duties.
Communicate. Communicate. Communicate.
Never abandon a candidate. Following up with candidates after their interview is a great way to show them that you are serious about the position and the hiring process. Make sure to thank each applicant for their time and tell them when they can expect an update on your decision.
Audit and Eliminate Unnecessary Steps
Every CRO wants to hire top-tier talent, and top-tier talent knows this. They know they have just as much power in the hiring process as the CRO. If you find yourself losing quality candidates during the interview process, it may be because your hiring system is more complicated or convoluted than your competitors.
Take an audit of your current application process and identify any bottlenecks or consistent drop-off points. Then weigh the benefit of this step (the information gathered, test of skill, etc.) with this drop-off point. Is it more important that your organization includes this step in the hiring process? Or should this step be dropped to improve your hiring rate?
5. Leverage New Technology
You should take advantage of the latest recruitment technology - utilizing online job boards, social media, and recruiting software to increase your reach and attract top talent. But, this isn’t the only space where technology investments can improve your clinical trial manager hiring efforts.
Providing your team with a strong technology platform can help improve efficiency, output, and employee quality of life. All of these, when known by a potential candidate, can help improve the likelihood of that candidate choosing to manage your clinical trial.
Clinical trial managers play a crucial role in the success of a trial, and it is important for CROs to ensure that they hire and retain the best talent available. By utilizing these five strategies, CROs can improve their CTM recruitment efforts and increase their chances of finding the best candidates for their organization.
Invest in Cloud Concinnity to Improve The Clinical Trial Experience
The Cloud Concinnity platform was built to meet the specific needs of clinical trial managers, CROs, and DSMBs. Our cloud-based system provides trial stakeholders with a centralized platform for patient information, communications, processes, and reporting onto a single, secure hub to improve their quality of life.
5 Clinical Trial Trends You Need to Know in 2023
Here are five trends that clinical trial managers must be aware of in the clinical trial space to maximize performance and improve the likelihood of a successful clinical trial outcome.
As 2022 draws to a close, one observation about clinical trials continues to ring true. Clinical trials are becoming more complex, and sponsors require more from their sites. Clinical trial managers must be aware of the latest trends in the clinical trial space to maximize performance and improve the likelihood of a successful clinical trial outcome. Below, we've compiled a list of the five clinical trial trends that you need to keep front of mind in 2023:
Five Leading Clinical Trial Trends in 2023
The Continued Rise in Digital Clinical Trials
As technology advances, so do the inclusion of these new technologies in managing clinical trials. More and more sponsors require that sites utilize digital technologies in their studies, from electronic case report forms (eCRFs) to cloud-based data capture systems.
Technology can only help streamline the trial process by reducing paperwork, keeping study teams up to date on the latest developments at all times, and eliminating costly errors.
This need for clinical trial oversight-specific software is why we created the Cloud Concinnity Platform. With Cloud Concinnity, your clinical trial managers and employees can quickly produce, share, and store critical clinical trial process management, data, and communications - all from the security and convenience of the Cloud. Learn more about Cloud Concinnity here.
Aggressive Trial Timelines
The global pandemic, supply chain issues, and the rise of inflation have caused clinical trials to be delayed for the past several years. To make up for this lost time, sponsors will be pushing out aggressive clinical trial execution timelines that require sites to work even harder than before to meet tighter deadlines.
Sites that can quickly adapt and keep pace with these increased demands are sure to be successful in the future. However, areas that need to adapt and maintain efficiency in an increasingly competitive climate may be left behind.
One crucial way for sites to stay ahead of the curve is by investing in new technologies and systems that can help them streamline their operations, improve patient recruitment efforts, and automate regulatory compliance more easily. For example, implementing electronic data capture can help sites speed up trial execution times by reducing paperwork and minimizing errors. Likewise, using a system with automation and workflows can help to standardize practices at scale. It also empowers your team with the ability to automate their workflows.
The Rise in Trial Consolidation
As clinical trials become more complex, sponsors will begin to look for ways to consolidate their studies and streamline processes to reduce costs and improve efficiency. Sites offering a full range of services across multiple indication areas will be highly sought after by sponsors as they work to consolidate their studies.
There are several reasons why clinical trial consolidation is becoming a popular choice in 2023.
The number of interventional trials grows, sponsors will look for ways to simplify their processes.
Sponsors may also be interested in reducing costs by consolidating studies into fewer sites to more efficiently manage their budgets.
Finally, with sophisticated data analytics capabilities and a growing network of clinical trial sites, sponsors will have no trouble finding the right location for their studies.
Sponsors looking to consolidate their trials in 2023 should choose sites that offer a full range of services across multiple indication areas. For example, some sites may be specialists in oncology or other specific diseases, while others may have more experience with drug development in general. By choosing a site that can offer multiple services, sponsors can reduce their overall costs and improve efficiency.
More Aggressive Screening Practices
To increase trial efficiency and improve data quality, sites will be required to screen patients more aggressively than before. This means that study teams must have efficient screening methods and invest heavily in technology solutions designed to support these procedures.
They must also have protocols in place that encourage engagement between the site, the patient, and the sponsor. This way, sponsors can be confident that trial data has been obtained from patients with a high likelihood of responding to treatment.
Trend Toward Personalized Medicine
With personalized medicine emerging as a major trend in healthcare, clinical trials are quickly adapting to this new approach. By understanding each patient's unique genetic makeup, sites can create more targeted interventions that improve outcomes and reduce the burden placed on patients during the study process.
By reducing unnecessary and duplicative trials, personalized medicine has the potential to save time, money, and resources for the broader healthcare industry. However, some barriers still need to be overcome before this model can fully take root in clinical trials.
For starters, much of the technology needed for personalized medicine is still nascent compared to other areas of healthcare delivery. The big data analytics required to reliably interpret patient DNA or genomics data is not yet readily available at all sites conducting research. In addition, sites that have access to this type of technology require highly specialized personnel – including genetic counselors, bioinformaticians, and biostatisticians– who are in high demand across many industries. As such, it can be challenging to recruit and retain these professionals, particularly if they are being asked to take on additional study sites or trials.
As these trends continue to emerge in the years ahead, sites need to stay up-to-date on all the latest developments so they can better prepare for what lies ahead. To learn more about how you can future-proof your clinical trial operations and thrive in 2023, be sure to schedule your Cloud Concinnity demo today!
8 Cybersecurity Tips for Managing Your Clinical Trials in 2023
Protecting clinical trial data is essential; a cyberattack can cause significant damage, delaying trial completions and introducing compliance, patience, and regulatory issues. Here are tips for managing cyber-safe clinical trials.
As a clinical trial professional, you already know that protecting your trial data is of the utmost importance. A successful cyberattack can cause significant damage, delaying trial completions and introducing compliance, patience, and regulatory issues. To help your clinical trials stay cyber-safe, we're sharing eight cybersecurity tips for managing your clinical trials.
What You Need to Know About the State of Cybersecurity
Before diving into the tips, let's look at the current state of cybersecurity and what you need to know about the cyberattack landscape.
95% of cybersecurity breaches are caused by human error. (World Economic Forum)
The U.S. was the target of 46 percent of cyberattacks in 2020, more than double any other country. (Microsoft)
Data breaches exposed 22 billion records in 2021. (RiskBased Security)
In 2021, nearly 40 percent of breaches featured phishing, around 11 percent involved malware, and about 22 percent involved hacking. (Verizon)
The top malicious email attachment types are .doc and .dot, which make up 37 percent; the next highest is .exe at 19.5 percent. (Symantec)
So, what does this tell us about cybersecurity?
As much as we want to believe the movies, most cybersecurity breaches happen due to human error. For a cybersecurity policy to be effective, it needs to consider this simple but powerful fact.
8 Ways to Protect Your Clinical Trials from Cyberattacks
Practice Proper Password Management
Set strong passwords for all of your accounts and change them regularly. Avoid using common words or phrases and personal information like your birthdate or address.
Also, avoid using the same password for multiple critical systems. If someone discovers a compromised password, they'll only have access to a single system.
Don't Share Your Login Credentials
Do not share your login credentials with other people, even if they are working on the same clinical trial project as you. If someone else has access to this information, it will be easier for cybercriminals to gain access to it.
Instead, add the individual to the needed system with their credentials.
Don't Be a Phishing Victim
Phishing is a common form of attack that involves sending email messages to trick people into revealing confidential information. Fortunately, there are several ways you can protect yourself from phishing attacks.
First and foremost, it's essential to be aware of red flags that might indicate phishing. For example, if an email message asks for sensitive information such as your username and password or asks you to click a link that then takes you to a page asking for the same kind of information, it could be a phishing scam.
In addition, if an email message includes poor grammar or spelling mistakes or seems suspicious in some other way, it could also be a phishing attempt.
Encryption = Security
Encrypt any sensitive data before storing it on the cloud, whether for clinical trials data management purposes or otherwise. This will help prevent unauthorized people from accessing this information, even if it is stored in the cloud.
Download Only What's Known
Never download software or apps from unauthorized websites or sources. Doing so could introduce viruses, malware, and other malicious programs onto your computer or mobile device.
These harmful programs are often hidden in the code of these downloads and can be triggered by users when they install them on their systems. If you aren't careful where you get your software from, you could introduce dangerous viruses and malware onto your system without even knowing it.
Avoid Public Wi-Fi
It’s always best to avoid using public Wi-Fi if you are working with any sensitive trail data. Public Wi-Fi systems have a greater chance of being compromised, which makes it harder for you to ensure a secure operation.
If you need to use public Wi-Fi networks for clinical trial management purposes, consider using a virtual private network (VPN) to encrypt your traffic and protect it from being monitored by cybercriminals on the same network.
Keep Your Systems Up to Date
Update your operating systems regularly to keep them up to date with the latest security patches to help protect against various threats and vulnerabilities. Installing the latest patches for your operating systems and software programs can help prevent malware threats from infecting your devices. Additionally, regularly updating all other device drivers can help keep your system secure and shore up vulnerabilities.
Be sure to Install a strong antivirus program that can help protect your personal data and devices from viruses and other threats. Lastly, using anti-spam and privacy features can also help you avoid phishing scams, which often attempt to steal your account information or compromise your phone's security.
Choose Secure Systems
At Concinnity, we believe that the future of clinical trial oversight is distinctly digital. This is why we've designed the Cloud Concinnity to include best-in-class security systems. Our cloud-based hub provides clinical trial managers with the tools they need to confidently and securely manage their clinical trials.
By following these cybersecurity tips for managing your clinical trials, you can ensure that you're doing everything possible to protect sensitive data from falling into the wrong hands. After all, your clinical trial data is vital to the success of your research projects, and you must take all necessary measures to protect it.
Request a demo to learn more about what the Cloud Concinnity platform can do for you.