How to Up Your Clinical Trial Data Monitoring Game
This post breaks down five steps to defining a successful clinical data monitoring strategy. Following the steps below can save your programs time and money and reduce risk to patients and the program overall.
Whether you are planning for the data needs of your Safety Review Committee (SRC), Clinical Trial Steering Committee (TSC) Data Monitoring Committee (DMC/IDMC/DSMB), Clinical Trial Advisory Board or CRO Oversight, consistent clinical data monitoring and oversight is critical to a successful clinical trial. Regularly capturing and monitoring your trial's clinical data puts your teams in the position to better understand your trial's status and empowers your team with the information they need to make real-time changes as necessary to optimize your program.
This post breaks down five steps to defining a successful clinical data monitoring strategy. Following the steps below can save your programs time and money and reduce risk to patients and the program overall.
5 Steps To Defining A Successful Clinical Data Monitoring Strategy
Step 1: Identify Your Most Important Data Points
Clinical trial data can be overwhelming, and it is easy to lose focus on the more essential aspects of the study. A successful data monitoring initiative starts by defining which data points are most important to the analysis, goals, and organizational strategy.
Consider early the information reporting needs of your oversight teams, both formal and informal, and ensure that the data is timely, reliable, and structured for easy interpretation. Don’t forget to consider relevant data emerging outside of your trial. Data and processes around them spelled out in DMC/DSMB charters and SOPs should be factored into your workflow.
These data points can fall into several categories, including:
Patient Count
Patient Recruitment
Completed Trials
Participating Facilities
Step 2: Establish Data Context Format
Clinical trials increasingly generate more and more complex data. That means more reading and more digesting. This makes developing a way to get a sense of things at a glance more difficult if not more important than ever. The length and content of data requirements for your oversight teams will vary. Committees should define their data content and format needs upfront, bearing in mind the importance of time, trends, and comparisons in their analysis.
Collecting critical data is essential, but data points themselves aren't a reflection of success or progress. Comparison data points help to provide context to your raw data points.
By comparing your captured data points to the right metrics, your teams can better determine if the clinical trial is on the path to success or if additional measures are needed to ensure a positive outcome.
Standard benchmark metrics include:
Historical Data & Trends
Industry or other Comparative Benchmarks
Internal Goals & Targets
As data presentation is defined, consider the value of providing summary information from past data reviews, interim data, protocol, amendments, recommendations, and any complex medical or statistical issues that influence data interpretation.
Step 3: Implement and Test a Data Capturing Methodology
What good is identifying your critical data points if you're unable to track and capture this information? Before signing off a data monitoring strategy, walk through the collection process for each data point.
Take the time to identify potential roadblocks and bottlenecks. Are you relying on manual actions to collect your critical data? Or is the collection automated? Where possible, eliminate any manual collection.
This will help to ensure a smoother data collection process while also eliminating the potential for human errors.
Step 4: Educating Your Teams on How to Analyze the Data
Think through your monitoring and oversight teams and identify the individuals that would most benefit from the clinical trial data you're monitoring. Take the time to ensure that they understand how to interpret and analyze the data points they are looking at to support and improve their area of responsibility.
Don’t presume that all participants come to the table ready, willing, and able to understand both the data and the process that they will be engaged in. Committee members, including DMCs, often have a wide range of experience and training. Left unaddressed, training needs will impact the cost and success of your program.
Training programs do not need to be formal or time-consuming. They do have to be well suited to the team or committee itself, so be sure to determine your teams’ training needs upfront.
Critical Components of a Data Training Process Includes:
An understanding of why the specific data points have been chosen.
A walkthrough of how the data is collected.
Guidance on how the data points relate to established goals/benchmarks.
Recommendations on how to analyze the data.
Workflows on any actions needed based on the data.
Key elements of data security, including the importance of protecting unblinded data
Step 5: Select The Right System for Your Data Needs
The clinical trial oversight ecosystem is complex and highly regulated. Unfortunately, the work of most monitoring teams and committees is mired in 20th-century point solutions - creating data and process insecurity and variability.
These systems are often home-grown, dated & generic, disconnected, highly manual, and error prone. They increase costs, risks & delays at a time when we need trials that are fast, safe, and efficient.
Clinical trial oversight depends on accurate, up-to-date information that is securely and quickly available to decision-makers. Centralizing this information securely in the cloud means the right people will have instant, 24/7 access to the right information they need.
What’s more, rather than trial updates needing to be sent over insecure connections, the information can be uploaded or updated automatically, when it’s ready, and is immediately available. At the same time, the information is more private and protected than ever because it is only being shared within a single, secure platform that has high-level privacy protection.
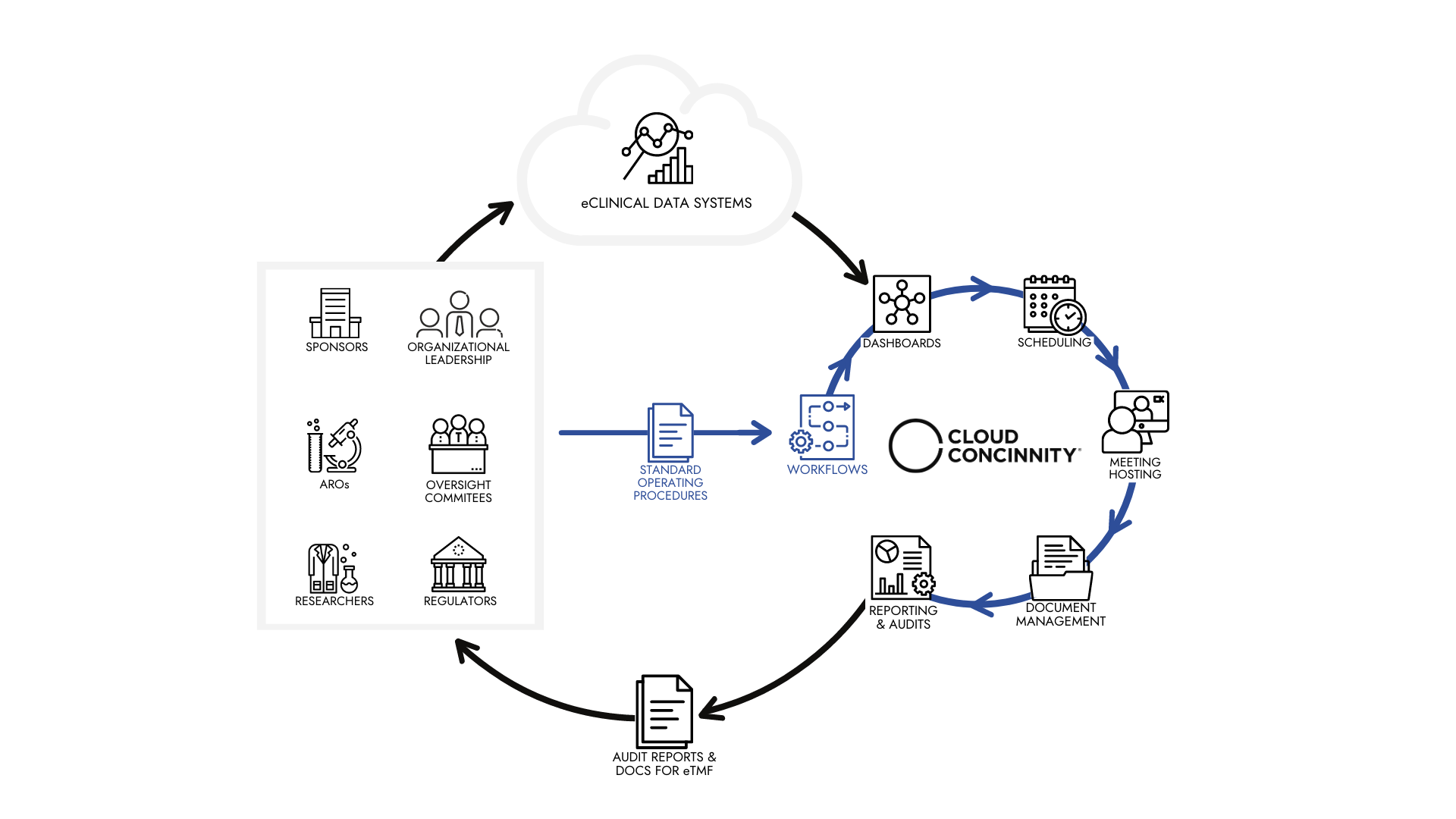
Improve Your Clinical Trial Data Monitoring with Cloud Concinnity
Cloud Concinnity is an integrated platform that centralizes, simplifies, and automates control of data, communications, process management & reporting while unlocking the value of big data. Systems like Cloud Concinnity can make it easier for your team to review the data collaboratively while providing a centralized hub for data capturing processes, measurement strategies, and reporting.
Data collection, monitoring, and reporting are critical to a successful clinical trial. By taking the time to define your data collection intentions, you're more likely to experience long-term clinical success.
The Concinnity Company Partners with Neuren Pharmaceuticals
The Concinnity Company partners with Neuren Pharmaceuticals provide next level clinical trial oversight management
“We are extremely happy to be Neuren’s partner in improving the lives of people with neurodevelopmental disabilities” said Nancy Falls, Concinnity Company CEO. Neuren Pharmaceuticals is developing new therapies for highly debilitating neurodevelopmental disorders that emerge in early childhood. Currently there are no highly effective or disease-modifying drugs approved for these conditions. Because these are serious medical conditions with unmet need, speed, as well as quality, is of the essence.
In addition to seeking regulatory fast tracks, Neuren sought to ensure superior security and efficiency in its clinical trial oversight process. In search of the best software solution for managing these important clinical trial oversight committees, Neuren found The Concinnity Company and its Cloud Concinnity® platform.
“Concinnity is extraordinarily functional and comprehensive clinical trial oversight software: a single, secure platform where all meetings, documents and processes can be centralized, automated, monitored and generate a complete audit trail.” said James Shaw, Vice President, Clinical & Regulatory Operations. “By partnering with outstanding CROs and Concinnity, we can be assured of speed, security and efficiency in our oversight processes.”
Neuren’s use of Concinnity’s innovative software supports the Data Safety and Monitoring Committees’ oversight of 3 trials testing the safety, tolerability and pharmacokinetics of NNZ-2591 and measures of efficacy in children and adolescents with 3 syndromes: Pitt Hopkins Syndrome, Phelan-McDermid Syndrome and Angelman Syndrome. Implementation of the Cloud Concinnity platform is expected to reduce any administrative burdens so that the DSMC can complete its work securely, thoroughly and quickly by improving meeting coordination, document sharing and general workflow practices.
“The future of clinical research depends on the kind of improvements in information and communications access and control, process consistency and tracking and outcomes transparency that are only achievable with smart technology,” said Falls. “This is especially true trials for our most vulnerable patients, ones that involve complex or adaptive trial designs, tight monitoring of dosing escalation, the analysis of multiple and complex data streams, and rigorous regulatory involvement.”
About Neuren:
Neuren is developing two new drug therapies to treat multiple serious neurological disorders that emerge in early childhood. The lead drug compound, trofinetide, is currently in a Phase 3 clinical trial for Rett syndrome and has completed a Phase 2 clinical trial in Fragile X syndrome. Both programs have been granted Fast Track designation by the US Food and Drug Administration (FDA). Neuren has granted an exclusive licence to ACADIA Pharmaceuticals Inc. for the development and commercialisation of trofinetide in North America. Neuren plans to initiate Phase 2 trials for a second drug candidate, for each of PhelanMcDermid syndrome, Angelman syndrome and Pitt Hopkins syndrome in 2022. Because of the urgent unmet need, all five programs have been granted “orphan drug” designation in both the United States and the European Union
Why Adaptive Design Clinical Trials Demand Stronger Digital Capabilities
As adaptive trial design emerges as part of the new normal, clinical trial oversight design must embrace smarter software like Cloud Concinnity® to adapt and thrive. For oversight teams, smarter software holds the key to addressing and solving challenges like 1) More Meetings, 2) More Decisions, 3) Tighter Timeframes, 4) More Complex Data, 5) More Data Streams and 6) Higher Stakes that come part and parcel with adaptive trial design.
Clinical trials are moving faster than ever, and decisions about starting, stopping, continuing or changing protocols are happening in real time, across geographies. It is critical to have a digital home that centralizes, simplifies and automates all of the crucial information, processes and tracking that can significantly impact the course of a program.
Conventional trial design and oversight has been pushed to evolve and change in the two years since the FDA first released their “Adaptive Design Clinical Trials for Drugs and Biologics Guidance for Industry,” and the Covid-19 pandemic has only hastened the shift, pushing adaptive trial design to the fore.
As adaptive trial design emerges as part of the new normal, clinical trial oversight design must embrace smarter software like Cloud Concinnity® to adapt and thrive.
Adaptive trial design takes clinical research to the next level, yet it presents some key challenges that come with speed and responsiveness. Challenges and demands like 1) More Meetings, 2) More Decisions, 3) Tighter Timeframes, 4) More Complex Data, 5) More Data Streams and 6) Higher Stakes come part and parcel with adaptive trial design.
For oversight teams, smarter software holds the key to addressing and solving many of these challenges. Here’s how:
6 Ways Smarter Oversight Software Meets the Demands of Adaptive Trial Design
1. More Meetings? Smarter Software Means Better Process Automation
Adaptive trial design means more data and more decisions to make — and that means more meetings to discuss and make those decisions. With an oversight team spread out across the world and across time zones, manually managing standard operating procedures and charter mandates can create significant delays. Using a platform that automates meeting management end to end gives administrators broad and deep capabilities like automated scheduling, invites, reminders, data aggregation and decision documentation making less work for everyone, and more time for deep thinking.
2. More Decisions? Smarter Software Lets You Stay Connected, Secure & Well-Informed
The data, information and consideration it takes to successfully oversee and manage an adaptive trial means lots of reading and questions. Every bit of that information must be kept confidential, which is easy with smart software that has the most advanced security protocols. What’s more, smart software can keep all communication during the decision-making process secure, so there’s no need to risk email, text, or video chats that could be hacked or leaked.
3. Tighter Timeframes? Smarter Software Centralizes & Streamlines Everything
We know that “Adaptive clinical trials can be completed sooner than trials with conventional (non-adaptive) designs,” so there is every reason to expect this faster, more nimble trial design to be widely adopted. And with the potential for speed comes tighter timeframes. Centralizing access, process, and outcomes means everyone on the oversight team need only check and stay updated on a single platform. Centralized means simpler, and simpler means faster. In addition, getting together as a team simultaneously to discuss everything, every time can be unrealistic. That’s why the secure, asynchronous communication that smarter software offers is critical. Share documents, have conversations and send messages across time zones and geographies.
4. More Complex Data? Smarter Software Simplifies Everything with a Master Dashboard
Adaptive trials deal with and generate more complex data than conventional ones. That means more reading and more digesting, but it doesn’t change the fact that we all need a way to get a sense of things at a glance. A single, secure hub for the aggregation, review and submission of data for oversight committees, sponsors & regulators makes that possible with a master dashboard and analytics. Customizable, global dashboards that aggregate key information within and across studies gives unprecedented visibility to key study results and metrics 24x7.
5. More Data Streams? Smarter Software Offers Powerful Data Integration
Adaptive trial design means more data streams, more information, and more decisions to make. A platform that is integrated across the eclinical ecosystem ensures data integrity from trial planning all the way through regulatory approval and beyond. Confidently create a single source of truth for your trial oversight activities that securely connects all points of information, from EDC to eTMF.
6. Higher Stakes? Smarter Software Delivers End to End Process Management
“Unlike conventional designs, where the learning typically occurs after the trial is completed, adaptive designs intend for continual learning as the data accumulate.” Continual learning and adaptive decision-making means more to manage, and that’s why the process management engine of smarter software is so critical. End to end process management is the ideal vehicle to power continual learning for a dispersed oversight team.
In the end, it’s clear that adaptive trial design is the way of the future, which means oversight teams will need to be more adaptive as well. Smart software is the only answer that lets teams step up to the next level while also maintaining or improving quality.
6 Data Security Benefits We Deliver for Clinical Trial Oversight
At Concinnity, our #1 concern is data security for clinical trial oversight.
As the world woke up to the dawning new normal of 2022, we saw thought leaders across healthcare pointing toward a digital future. Aman Khera, the Global Head of Regulatory Strategy at Worldwide Clinical Trials, cited “Digital Health” and digital transformation as the top trends shaping clinical trial regulations in 2022, noting that everyone from the WHO to the FDA and EMA are looking closely at how digitization tools should be used, and at how data is stored and used. BCG recently proclaimed that healthcare’s new reality is dynamic, digital, and here to stay.
Security for digital data and communication is what makes the digital future of clinical trial oversight possible.
At Concinnity, we have focused on data security from day one. We know that digital security for all stakeholders at every level of the process — from study sponsors and CRO’s all the way down to each individual study participant — is an inherent and primary need to pave the way for this digital future. It is our security features that make our platform a game-changer, by making Cloud Concinnity the most secure platform available for clinical trial oversight management.
Concinnity is structurally designed to adhere to the highest standards of data security, including the special protections required by law for health information by HIPAA and other related regulatory requirements.
Here are the top 6 data security benefits we deliver for clinical trial oversight:
Separate Workspaces for Blinded and Unblinded Study Teams
At the very core of clinical trial oversight is the need for certain iron-clad data separation. To ensure this, Cloud Concinnity creates a “physical” separation of blinded and unblinded data that eliminates the risk of any stakeholder sharing unblinded information. In short, we make it virtually impossible for oversight data accidents to happen.
Superior UI/UX (usability) that Improves Security
The simple, clean, intuitive user interface that makes Concinnity a joy to use also makes it more secure. Our superior UI/UX and deep structural platform integrity dramatically reduces any risk of user error and of any individual using the software incorrectly or accidentally going around the system.
Patent Pending Workflow Engine that Safeguards Processes and Data
We understand that procedures and processes are as important to data security and compliance as software structure. Manual processes by nature are subject to human error and vulnerable to the loss of institutional knowledge during staffing transitions. Our proprietary workflow engine includes features that easily automate best practice processes across a portfolio of studies, protecting data and reducing the risk of variability or non-compliance.
Comprehensive Oversight Solution Protects Patient/Sponsor Data
We built capabilities and productivity integrations that allow participants to do all of their clinical trial oversight work on a single, secure platform. This eliminates the significant risk introduced by transmission of sensitive information into and out of various non compliant applications, from email on multiple servers to scheduling, video conferencing, eSignature and document editing apps where data insecurity is rife.
Integrated Platform Ensures Data Integrity from trial planning all the way through regulatory approval and beyond
Confidently create a single source of truth for your trial oversight activities that securely connects all points of information, from EDC to eTMF. Robust data integrations remove the risk of data loss, leakage, and unblinding. Automated data integrations protect clinical trial data and security on the way in, eliminating the need for manual or episodic uploads. Direct integrations with reporting and document management systems protect critical decisions and auditability on the way out.
Regulatory Compliance That Goes Above and Beyond
Cloud Concinnity is compliant with all 21 CFR Part 11 regulations, and maintains a host of further regulatory compliance credentials. We maintain SOC2 compliance, and use further information security elements or setup elements like 2-factor authentication for login, plus role-based and configurable permissions to control access to specific data/content/information.
The bottom line? Cloud Concinnity is by far the most secure choice to manage clinical trial oversight operations, and the only clinical trial oversight software on the market equipped to fully make the digital future of clinical trial oversight possible.
The Year Ahead: Big ideas for the future of clinical trial oversight.
What does it take to build the future? What mindset does a team need to create breakthrough clinical trial oversight software that will impact millions of patient outcomes?
It takes innovation, commitment & the courage to lead.
While the world continues to wrestle with Covid, we at Concinnity have grown even more committed to our role in bringing life changing treatments to market more quickly, safely and efficiently. The future of clinical trial oversight is undoubtedly digital, and utilizing smart software to facilitate information access, process control and outcomes visibility is imperative. 2022 will be a pivotal moment for our industry. We feel like it is our moment. Why? As one of our team posted this week:
“The challenges of the clinical trial oversight ecosystem feel like they were created for us; a place where we can be innovative and idealistic where the ceiling will be created and determined by us.”
To start off 2022, we asked ourselves: What excites us about the future of clinical trial oversight? There were too many good ideas and insights to publish everything, so we’re offering a condensed look behind the scenes at what our team is thinking.
Here are a few of the big ideas driving our work as the calendar turns to the new year:
DOING OUR PART TO HELP HEAL THE WORLD
Given that the revolution/evolution of the clinical trial ecosystem continues, we’re excited and motivated by the impact Concinnity's technology can make on helping to heal the world. We'll be providing crucial services to pharmaceutical companies and DSMBs that will be called upon to make faster decisions about the safety and efficacy of clinical trial results.
MAKING A DIRECT HUMAN IMPACT
We are proud to be part of an industry that is making a direct impact on the human race. One of our customers is the first pharmaceutical company ever to show positive results in a phase 3 neurological disorder. We are delighted to be working with such passionate, innovative professionals. Need we say more?!?
HELPING CUSTOMERS DO MORE WITH LESS
In the face of shrinking support resources across industries, we're poised to enable our customers to do more with less.
MAKING TECHNOLOGY THAT CHANGES THE WORLD
As Covid becomes endemic and more a part of a new normal, we anticipate seeing a rapid uptick in drug trials. We see this year as the time when both sponsors and the CROs recognize the need to use technology to make the management of DMCs both more secure and more efficient.
In the year ahead, we know that changes and challenges to the industry will continue. But the good news is, we are powering the smart, digital future of clinical trial oversight with the most functional and comprehensive clinical trial oversight software on the market. And thousands of women and men across the globe are committed to the success of trial operations in the midst of unprecedented hardship.
Our belief in the power of our collective hard work and innovation gives us faith in our shared future. We believe we will look back on 2022 as a year of great progress. Let’s lead and innovate together!
Learn more about how Cloud Concinnity can support your clinical trial oversight.
Want more forward-looking insight? Here’s a sample of our deepest insights on what 2022 will bring:
A Look Ahead: 4 Clinical Trial Oversight Trends That Will Guide 2022 and Beyond
Thinking Ahead: 5 Post-Pandemic Priorities for Clinical Trial Oversight
Our 3 Top Articles from 2021: A Year End Review
As the year comes to a close, we wanted to share a few of our favorite insights from this past year. We invite you to look back with us, and take a moment to pause and reflect on what challenges and changes the year has brought for you.
2021 has been a year of rapid, seismic change in clinical trials and clinical trial oversight. A year ago as we approached the end of 2020, COVID vaccines were only just starting to get approved. World travel was still dramatically restricted. Joe Biden was not yet inaugurated. In short, it was a lifetime ago.
As a world and as an industry, we have come together and made it through.
At Concinnity, we have grown even more passionate about our role in bringing life changing treatments to market more quickly, safely and efficiently. The future of clinical trial oversight is undoubtedly digital, and utilizing smart software to facilitate information access, control process and highlight outcomes is imperative.
As the year comes to a close, we wanted to share a few of our favorite insights from this past year. We invite you to look back with us, and take a moment to pause and reflect on what challenges and changes the year has brought for you.
1. 4 CRITICAL CONSIDERATIONS FOR CLINICAL TRIAL OVERSIGHT
“In this age where digitally enabled productivity gains are accelerating the fourth industrial revolution across industries, it means identifying and making critical changes. Boston Consulting Group has found that 70% of digital transformations end in failure, and that just 30% of companies are succeeding. So what does it take to make sure you and your oversight teams are in that top third?”
In this post, we explored the most pressing issues for everyone in clinical trial oversight to grapple with. Do you agree with where we landed?
2. 5 NEW WAYS CLINICAL TRIAL OVERSIGHT PRACTICES CAN RISE TO TODAY’S CHALLENGES
“Today’s turbulent environment has precipitated an unprecedented level of pressure on sponsors, CROs, and the ecosystem of oversight committees needed to create and conduct a clinical trial. The accelerated rate of change means that if these key stakeholders aren’t effectively engaging key issues of strategy and risk, they are competitively handicapped at best and, at worst, in risk of crises. We look at some of the old processes that are being challenged -- and how Cloud Concinnity can facilitate critical transformation.”
Here, we focus on the best practices and tactics that can make a huge difference in how effective processes can be. It’s a perfect list to check if your oversight teams are doing everything they can.
3. HOW INNOVATION MUST ADDRESS THE ONGOING IMPACT OF COVID-19 ON CLINICAL TRIAL OVERSIGHT
“It is clear that innovation driven by digital transformation will drive the future of clinical trial oversight. Not only will the coming years be challenging to navigate, they will also push all of us to work together to create a truly innovative clinical trial oversight ecosystem.”
Finally, we look at the biggest issue of the year – COVID-19 – and how it has impacted the industry. Whether long-term or short-term, COVID has changed how we do almost everything.
In the year ahead, we know that changes and challenges to the industry will continue to demand innovation. We are committed to powering the smart, digital future of clinical trial oversight. Cloud Concinnity® is the most functional and comprehensive clinical trial oversight software on the market: a single, secure platform where all meetings, documents and processes can be centralized, automated and monitored, generating a complete audit trail., We look forward to working with you and your team in the new year.
Learn more about how Cloud Concinnity can support your clinical trial oversight
10 Remarkable Clinical Trial Stats Illuminating the Future of Oversight
Today we zoom out and take a look at some of the statistics behind clinical trials and oversight work to see how devastating delays and disruptions can be, opportunities to address those problems, and explore what all of this may reveal about the future of clinical trial oversight.
As the year comes to a close, we are reminded how deeply important great clinical trial oversight is to both the clinical trial ecosystem and to the world. COVID has pushed us all to be more thoughtful, resilient and efficient in the work we do, and what we are all living through is truly a turning point for how clinical trials happen.
Improving clinical trials and clinical trial oversight for the benefit of every stakeholder -- from sponsors all the way through to patients -- is one of the most compelling opportunities of our time. Nothing less than the future of healthcare is on the line, and every step in the process counts. As creators of breakthrough trial oversight software, we are committed to making clinical trial oversight more efficient and effective, and we see ways to support stakeholders at every level of the process.
Today we zoom out and take a look at some of the statistics behind clinical trials and oversight work to see how devastating delays and disruptions can be, opportunities to address those problems, and explore what all of this may reveal about the future of clinical trial oversight.
CLINICAL TRIAL OVERSIGHT STATISTICS -- THE BIG PICTURE
Providing safe and effective treatments has never been more important, and that demands focus on improving the effectiveness of oversight. In the short term, we continue to see the critical importance for addressing COVID-19, but in the longer term it’s clear that we must address the growing healthcare needs of an aging global population.
1967: The year that an NIH external advisory group “first introduced the concept of a formal committee charged with reviewing the accumulating data as the trial progressed to monitor safety, effectiveness, and trial conduct issues in a set of recommendations to the then-National Heart Institute.” While DMC’s had been a part of some clinical trials since the 1960’s or earlier, it has been about 55 years since they were formally recommended by the NIH. (source: https://www.fda.gov/)
$198 billion: Estimated global spending on Pharma R&D in 2020. (source: statista)
18,582: The number of drugs currently in the R&D pipeline as of October 2021. (source: statista)
Oversight innovation through smart software means that the hundreds of billions spent in drug development will be used as efficiently and effectively as possible. The post-COVID oversight world will live online, and we are already shaping how it can build the future on top of the first 50 years.
CLINICAL TRIAL OVERSIGHT STATISTICS -- COST OF DRUG DEVELOPMENT
It is shocking just how costly study delays and disruptions are, and a major challenge is how normalized study disruptions are. Smart software in clinical trial oversight will continue to grow and expand as an effective tool for mitigating those risks and costly delays and disruptions.
$2.6 billion: The average cost of developing a drug in the United States, as of October 2021. (source: statista)
$600,000 and $8 million: What each day of trial delay during a product’s development and launch can cost sponsors. (sources: pharmafile and antidote)
9 out of 10: Number of trials that require the original timeline to be doubled in order to meet enrollment goals. (source: antidote.com)
Smart software is an easy-to-implement, powerful tool for efficient oversight that has immediate impact on data security and oversight committee communication. There is no going back to how things were before COVID, and we are thrilled to be driving innovation across the clinical trial oversight ecosystem that will impact stakeholders and processes across the board.
CLINICAL TRIAL OVERSIGHT STATISTICS -- THE IMPACT OF COVID
New study launches took a massive hit in 2020 when the initial COVID outbreak happened, and the ripple effect is still making an impact across the clinical trial oversight ecosystem right up through the end of 2021. What we see is that COVID-19 slashed the number of active studies, pushing everything back, and making the queue for new studies even longer.
57%: The percentage of studies initiated in the U.S. from February to May in 2020 vs. what would have been expected had the pandemic not occurred. (sources: pubmed.ncbi.nlm.nih.gov and nature.com)
77%: The percentage of studies initiated outside of the U.S. from February to May in 2020 vs. what would have been expected had the pandemic not occurred. (sources: pubmed.ncbi.nlm.nih.gov and nature.com)
40,000: Number of already-enrolled patients impacted by the disruption of trials by COVID-19 (source: centerwatch.com)
44%: Percentage of the more than 2,600 clinical trials stopped in the first five months of the 2020 that cited the COVID-19 outbreak as the cause. (source: centerwatch.com)
Moving forward, the clinical trial ecosystem will need newer, better and ever more efficient ways to oversee studies, both to make up for lost time and to manage the ever-growing number of new studies. Smart software is the answer, and the way forward.
With all of this in mind, shouldn’t your clinical trial oversight team use the best possible oversight software?
Learn more about how Cloud Concinnity can support your clinical trial oversight
How DSMB Expert Teams Create Magic
DSMBs and other expert clinical trial oversight teams can go above and beyond in their work to create magic by leveraging smarter software and the advantages and opportunities it can offer.
In the decades since DSMB’s were first established, their makeup and structure may have changed, but their mission has stayed the same: To protect the safety of patients, ensure the integrity of data and determine treatment efficacy in clinical trials. To do this, DSMBs must create an important sense of trust during the drugs and treatment development process -- a sense of trust that will ripple out to stakeholders in every direction.
What was once a team of experts operating behind the scenes has been thrust into the spotlight during the pandemic. Indeed, DSMBs today not only need to perform their duties, but they need to do them in an unbiased, consistent and documented way that will hold up to public scrutiny. So, how can clinical trial oversight teams create that magical sense of trust in a study and its outcomes that translates numbers and research into something everyone can put their faith in?
Today, DSMBs and other expert clinical trial oversight teams can go above and beyond in their work to create magic by leveraging smarter software and the advantages and opportunities it can offer.
1. LEVERAGE NEW STRATEGIC COMMUNICATION NORMS AND EXPECTATIONS TO BUILD NEW STANDARDS FOR EXCELLENCE & TRUST
When a DSMB is equipped with state of the art cloud software for communication and data management, their work can go from “keeping up” with the pace of the study to driving greater speed and safety and process transparency to their important work. DSMBs so levered with smart technology can set new standards for excellence and trust. Smart software allows DSMB members to securely communicate between meetings, making sure that everyone is updated along the way and that people are ready to make decisions faster.
This means the DSMB members and ad hoc support can begin the study knowing that they will all be asynchronously available to each other throughout the study, not just during scheduled meetings. By setting the expectation of strategic conversations between meetings, the continuity of the study oversight can be supported.
2. ENGAGE AD HOC SPECIALISTS TO SUPPORT AND STRENGTHEN OVERSIGHT WITH ASYNCHRONOUS WORK IN REAL-TIME
In addition to the “1) One or more experts in the clinical aspects of the disease/patient population being studied, 2) One or more biostatisticians, and 3) Investigators with expertise in current clinical trials conduct and methodology” that the NIH specifies every DSMB must include, the NIH also states that ad hoc specialists can be brought in to advise at any time. Whenever possible, this is an excellent option, and one of the major obstacles has always been geography.
The pandemic has forced us all to work differently, and with the normalization of remote work, so too are we seeing an opportunity to normalize and leverage ad hoc specialists for DSMBs. Oversight teams who want to bring in ad hoc specialists to support and strengthen their oversight can use smarter software to offer secure access to study data and secure communication with the rest of the team. A single, integrated, cloud-based platform supports virtual and asynchronous work in real-time.
3. DEEPEN AND SUPPORT APPROPRIATE EXCHANGE BETWEEN CRO/SPONSOR AND DSMB
Smart software, platform architecture, as well as role-based permissions, can facilitate and protect communications for faster, safer clinical trials. Because every DSMB is part of a study that has a CRO and/or a sponsor, there will need to be communication between the DSMB and these organizing entities. But those communications also need to be highly regulated to protect data and independence. All participants must have direct, easy access to what they need, when they need it, but must be blocked from what they should not see.
At the same time, smarter software can offer the opportunity to make communication and updates more frequent and effective from the DSMB to the CRO/Sponsor. Because study data and communication will live in a central, secure location that needs less upkeep, this opens up opportunities for the DSMB to communicate in new and supportive ways. Indeed, when the work of the DSMB for clinical trial oversight gets easier, everyone’s work gets easier -- and that is great for patients and drug development.
Smart software allows for faster thinking at a time when we need it most. At a time where we are all still adjusting to post-pandemic norms it gives teams a chance to adapt to these new normals. But most of all, the forward-thinking teams that embrace smart software for their work can create the much-needed trust in the process and the invaluable treatment fruits of their efforts. This is the real magic. Can your trial oversight team create it?
Click here to access our essential guide to the future of clinical trial oversight
3 Advantages of Cloud Transformation for Clinical Trial Oversight
The opportunity for digital cloud transformation to impact the clinical trial oversight ecosystem has arrived, offering myriad advantages to sponsors, CROs and DSMBs. Those who start now can still be ahead of the digital curve. Today, we look at 3 advantages that cloud transformation offers to clinical trial oversight players, if they anchor their transformation in enterprise, integrated platforms.
As the world adapts to the new normal of life in the wake of COVID, thought leaders are looking deeply at how industries are transforming through the digital cloud, AI and other smart technologies. In healthcare, the digital cloud transformation is already well underway, as evidenced by this analysis of What [healthcare] payers and providers can learn from successful cloud transformations in other industries.
The opportunity for digital cloud transformation to impact the clinical trial oversight ecosystem has arrived, offering myriad advantages to sponsors, CROs and DSMBs. Those who start now can still be ahead of the digital curve.
Today, we look at 3 advantages that cloud transformation offers to clinical trial oversight players, if they anchor their transformation in enterprise, integrated platforms.
1. FACILITATED ACCESS TO INFORMATION & COMMUNICATIONS EMPOWER DSMBs TO MOVE FASTER & MORE SECURELY
Clinical trial oversight depends on accurate, up-to-date information that is securely and quickly available to decision makers. Centralizing this information securely in the cloud means the right people will have instant, 24/7 access to the right information they need.
What’s more, rather than trial updates needing to be sent over insecure connections, the information can be uploaded or updated automatically,when it’s ready, and is immediately available.
At the same time, the information is more private and protected than ever because it is only being shared within a single, secure platform that has high level privacy protection.
Truly, we are seeing a revolution in the way a DSMB can do its work.
2. CONTROLLED PROCESSES BENEFIT COMMITTEE EFFICIENCY AND CONSISTENCY
Hosting clinical trial oversight processes in an integrated, cloud-based platform will help formalize and automate many steps of the DSMB workflow. Scheduling meetings will be streamlined, creating reports will be simplified and access to information will be centralized. In short, what has always taken a series of phone calls or email threads can now be done with a few clicks -- or automated altogether.
The oversight administrator will have an easier way of creating repeated, trusted processes for members; the DSMB as a team will be able to complete key tasks on their own time while engaging in asynchronous conversations, and every stakeholder involved in the study oversight will have a simple, central authority available for access when they need it.
Processes will drive the timeline, not the schedules of individual people. A cloud based integrated platform will make everyone’s life easier, and the oversight of every study more efficient.
3. TRANSPARENT OUTCOMES PROVIDE BETTER RISK MITIGATION
A further advantage of cloud-based integrated transformations is that all outcomes are much easier to track and are more transparent, allowing for improved risk mitigation. When information and processes live in the cloud, they are easily trackable, which gives administrators the unique opportunity to analyze activity after the fact. Over time, administrators will be able to observe and address patterns and optimize the risk management of a study, accelerating the overall risk mitigation processes.
On an even broader scale, CRO’s and sponsors will be able to see and review aggregate data within and across studies and portfolios, offering insight into best practices that help manage risk in studies currently underway, and inform next practices for studies currently in the pipeline. Overall, risk mitigation will benefit from the transparency of outcomes that promote improvement for all.
The future of clinical trial oversight is powered by smart cloud transformation that is happening across industries and is already changing every corner of the healthcare industry.
Is your oversight team ready to be part of this cloud transformation?
Learn more about how Cloud Concinnity can support your clinical trial oversight
How Innovation Must Address the Ongoing Impact of COVID-19 on Clinical Trial Oversight
COVID-19 has deeply impacted every aspect of clinical trial oversight, but we are only now beginning to explore its ongoing impacts and effects. In looking at “the next wave of healthcare innovation,” McKinsey zooms in on how innovation can transform longstanding inefficiencies, and how technology is the key to unlocking a future that accelerates that innovation.
It is clear that innovation driven by digital transformation will drive the future of clinical trial oversight. Not only will the coming years be challenging to navigate, they will also push all of us to work together to create a truly innovative clinical trial oversight ecosystem.
Today, we highlight 3 areas where innovation is called on to address the ongoing impact of COVID-19 across the clinical trial oversight.
1. OPTIMIZE PROCESSES AND REDUCE COSTS TO INCREASE PATIENT BENEFITS
While research and study protocols typically only change over long periods of time, when COVID-19 arrived in 2019, these protocols and study models had to change literally overnight. The answers about how best to manage oversight of a study in today’s environment are still far from settled.
Oversight processes need to be optimized for efficient, effective remote work. Optimizing oversight processes will support getting new drugs to market faster, thereby positively impacting patients down the line. At the same time, optimizing oversight processes for remote work creates countless opportunities for reducing costs. This is where oversight innovation -- driven by smart technology -- can impact the bottom line of every stakeholder in the process.
2. STAY AHEAD OF THE PACK TO GET A JUMP ON FUTURE INNOVATION
Understanding that smart technology will drive the necessary oversight innovation is one thing. Being the group who researches, understands, and chooses the right solution is the real step that will set you apart from the pack. And staying a step ahead of competitors is critical. We have a whitepaper full of deep insight into exactly why and how.
Getting started in 2021 on the innovations that will serve you in 2022 and beyond is the key to getting ahead of the pack. It is tempting to employ short term tech solutions that work “for now,” but it is the stakeholders who choose to plan for the ongoing impact of COVID-19 who will thrive in the next chapter of clinical trial oversight.
3. STAYING ON TOP OF REGULATORY AND COMPLIANCE CHANGES TO SET THE TONE
Smarter software and innovative approaches to the impact of COVID-19 does not stop with optimized processes and market advantages. COVID-19 is also impacting the regulatory and compliance environments of every industry.
We need innovative ways for oversight committees to stay updated personally and as a group on these changes. And, we need innovative ways for remote oversight teams to quickly get on the same page about how study protocols and other procedures and requirements for final documents are changing. All of this is best accomplished with smart technology.
The future of clinical trial oversight will be driven by innovation driven and digital transformation. will drive the future of clinical trial oversight -- it’s the only way to stay on top of the ongoing impact of COVID-19.
How is your team jumpstarting oversight innovation in your ecosystem?
Click here to learn more about how
Cloud Concinnity can support your clinical trial oversight
Thinking Ahead: 5 Post-Pandemic Priorities for Clinical Trial Oversight
In their ongoing series, McKinsey talks about “the next normal” and the importance of “emerging stronger from the coronavirus pandemic.” We at Concinnity are committed to doing our part to make sure that the clinical trial ecosystem can do just that — emerge from the challenges of the coronavirus pandemic with even greater focus, speed, security and scale than before.
Today, we look at the 5 priorities that McKinsey calls out for CEO’s as we build the next normal, and think about how these recommendations apply to the emerging imperatives for the clinical trial ecosystem.
1. HARNESS THE CLOUD
Responsible, forward-thinking oversight must be built around the cloud. McKinsey writes that “By 2030, there could be $1 trillion at stake — and it’s likely that early adopters will win the lion’s share.” In clinical trial oversight, we believe the same will be true — the lion’s share of the benefits, by way of speed and scale for new drug development, will go to those who leverage cloud technology to supercharge the effectiveness of their oversight teams and committees.
2. RECONSIDER SUSTAINABILITY
Here, the focus is on how clinical trial oversight can do its part to adapt to climate change. At first blush, this feels largely unrelated. However, building operations around cloud technology, ideal for remote work during the pandemic, will also make it easy for oversight teams to reduce their travel and commutes for the long term. Indeed, cloud technology is a prime example of how a combination of innovation and necessity can accelerate growth that is good for the planet, the people, and the bottom line.
3. LEVERAGE YOUR TALENT
While oversight teams are often not together for the long term, that doesn’t mean that being part of trial oversight work can’t be an efficient and educational experience for all participants. Access to an ever-growing library of thought leadership, best practices and automation can help everyone on the team optimize their time, get more done and enjoy the work. Cutting non-value added tasks and work from the process frees up time for thought, higher level taks and learning. Oversight committee members and support teams alike are precious resources to be used more wisely through better systems, content and processes.
4. EMBRACE SPEED
The promise of cloud technology to support faster, more efficient work is unparalleled. The pandemic has pushed us all to adopt the agile, iterative work methods that will serve us even more beyond the pandemic. When professionals can work from anywhere, they work faster. When mundane tasks can be automated, there is more time (and energy) for the hard work, which means it gets done faster and better. And when clinical trial oversight is faster and more focused, breakthrough drugs and therapies can get to market and serve more patients.
5. RECONNECT WITH PURPOSE
While geography once served as a limiting factor for finding mission-aligned oversight team members, cloud technology enables finding and engaging the optimal clinical trial oversight talent. And finding meaning in work is no longer a “nice to have”. It can be a driving factor in how oversight teams are put together. This will go a long way toward aligning everyone involved around a shared purpose, supporting the speed and scale aspects of new drug development.
As McKinsey has pointed out, some priorities will rise above the rest as we move into a new reality beyond the pandemic. These same priorities can focus and drive the post-pandemic priorities for every stakeholder in clinical trial oversight.
Are you ready?
Learn more about how
Cloud Concinnity can support your clinical trial oversight.
Concinnity Featured As Part of the Venture Atlanta Showcase
We are honored to announce that Venture Atlanta has selected us as one of the top technology companies in the Southeast, and that Concinnity will be featured at the upcoming Venture Atlanta Conference as a Software Showcase company.
We are honored to announce that Venture Atlanta has selected us as one of the top technology companies in the Southeast, and that Concinnity will be featured at the upcoming Venture Atlanta Conference as a Software Showcase company.
Venture Atlanta is an annual collaboration between the Atlanta CEO Council, Metro Atlanta Chamber, and the Technology Association of Georgia (TAG) and one of the premiere technology innovation event in the Southeast.
Allyson Eman, CEO of Venture Atlanta, says:
"Venture Atlanta has become the authority for recognizing technology innovation across the Southeast and beyond, connecting the best and brightest innovators with top-tier, national investors and other leaders in our tech ecosystem."
Venture Atlanta has been selecting, supporting and connecting the most promising tech companies and bringing in top investment firms from across the nation to meet them for 14 years. The annual conference has helped 500+ companies launch and raise $6.5 billion in funding to date.
Nancy Falls, our CEO, says:
"We are delighted to receive this recognition. Technology enabled productivity gains are the future. Venture Atlanta gives us the opportunity to advance this future where we need it most. The needs of clinical trial boards and committees parallel those of other governance, risk and compliance teams. But, as the pandemic has made clear to most of us, these are some of the most complex and pressing needs of our time. Supporting clinical trials is the highlight of our work."
We are thrilled to be part of Venture Atlanta 2021. COVID-19 has disrupted almost every aspect of clinical trial management and oversight. We know that smart software will power the emerging future of clinical trial oversight.
Cloud Concinnity® provides the most functional and comprehensive clinical trial oversight software on the market: a single, secure hub where all meetings, documents and processes can be centralized, automated, monitored and generate a complete audit trail. We are proud that Cloud Concinnity® enables developers of new therapies to bring their treatments to market faster, at a lower cost all while reducing risk to Sponsors, Clinical Research Organizations (CROs) and patients.
Click here for the full press release.
Learn more about how Cloud Concinnity can support your clinical trial oversight.
4 Critical Considerations for Clinical Trial Oversight
In this age where digitally enabled productivity gains are accelerating the fourth industrial revolution across industries, it means identifying and making critical changes. Here are the 4 critical considerations for your clinical trial oversight team to discuss today.
Researchers, sponsors, and CRO’s know that the time to think deeply about clinical trial oversight is before a trial starts, not after. We need only glance at the recent headlines about DSMBs to see how important it is to stay ahead of the curve.
Boston Consulting Group has found that 70% of digital transformations end in failure, and that just 30% of companies are succeeding. So what does it take to make sure you and your oversight teams are in that top third?
In this age where digitally enabled productivity gains are accelerating the fourth industrial revolution across industries, it means identifying and making critical changes.
Here are the 4 critical considerations for your clinical trial oversight team to discuss today.
1. SHARED EXPECTATIONS & NORMS DRIVE CLINICAL TRIAL OVERSIGHT SUCCESS
Communication expectations and norms must be set for the DSMB and other stakeholders before the study begins. Especially in the emerging post-pandemic world where the rules are being rewritten for everyone, clear expectations are paramount.
This means getting involved, from the top and from the beginning, with how DSMB teams are interacting. Beyond the required protocols, is everyone who needs to be communicating already communicating? As the sands shift beneath our very feet, we all need to align around resetting norms.
2. SOFTWARE TRAINING & SUPPORT MUST UNDERSTAND CLINICAL TRIAL OVERSIGHT
As a smart software company, we believe in the power of technology. And because we know that shifting habits is hard, we believe even more in robust support and training.
Researchers, sponsors, and CRO’s must ask: Does everyone on the DSMB know how to use the software? Have all support staff and experts been properly onboarded and trained? Does everyone know who to call if there is a software question? When shifting the habits and processes, ongoing support and training are critical. Make sure it’s set up in advance for your team.
3. PUT THE DIGITAL TRANSFORMATION ODDS IN YOUR FAVOR FOR REAL CLINICAL TRIAL OVERSIGHT
Boston Consulting Group has found that the majority of companies attempting a digital transformation fail. So what does this mean for the clinical trial oversight ecosystem? It means that the odds are stacked against us all -- unless we make educated changes. BCG has also identified the key factors for flipping the odds of digital transformation success in your favor, and they all deal with having a clear strategy that starts from the top, stays agile, and involves measuring and monitoring along the way.
In the complex clinical trial oversight ecosystem, setting the right tone from sponsors, researchers, and CRO’s is critical, and that starts with the earliest conversations about the next study.
4. MEASURE YOUR RESULTS FOR BETTER CLINICAL TRIAL OVERSIGHT
To improve, we must measure. We all know this, yet it remains difficult to put into action. This simple paradox makes the necessity of ongoing and targeted outcome measurement absolutely critical for improving clinical trial oversight.
The good news is that implementing a single, secure smart software hub will do both. It puts processes and protocols in place that will support better outcomes, and it implements automated measurement of outcomes that will make it easy to show just how much your DSMB is contributing to improved outcomes, from improved clarity of communication to rapid efficiency of creating reports and sharing data.
--
The time has already arrived for improved clinical trial oversight. The question is not whether it is possible, but whether you are ready to step into the critical considerations that will make the future of the clinical trial oversight ecosystem as good as it possibly can be.
Let’s do this.
Talk to someone today about how your team can step into
the future of clinical trial oversight.
A Look Ahead: 4 Clinical Trial Oversight Trends That Will Guide 2022 and Beyond
We know that the fourth industrial revolution is already underway, as we are seeing the automation of business processes using modern smart technology all around us. Pair this trend with the understanding that remote work -- and especially remote committee meetings for oversight -- is here to stay, and a clear picture of a digitally enabled future for clinical trial oversight emerges.
So what does this mean for researchers, sponsors, and CRO’s?
The clinical trial oversight ecosystem is changing right before our eyes -- and fast. In an article titled The Next Normal Arrives: Trends That Will Define 2021—and Beyond, McKinsey calls out a range of shifts that will change the way we all do business.
Which one should hit home for everyone involved in clinical trial oversight?
Digitally enabled productivity gains accelerate the Fourth Industrial Revolution
We know that the fourth industrial revolution is already underway, as we are seeing the automation of business processes using modern smart technology all around us. Pair this trend with the understanding that remote work -- and especially remote committee meetings for oversight -- is here to stay, and a clear picture of a digitally enabled future for clinical trial oversight emerges.
So what does this mean for researchers, sponsors, and CRO’s?
1. CLINICAL TRIAL OVERSIGHT TREND #1: SMARTER SOFTWARE WILL DRIVE PRODUCTIVITY
Many oversight teams and committees are already using technology to some degree, and this trend will only continue. The big change? It’s that using these technologies -- especially smart software designed specifically for clinical trial oversight -- can rapidly increase the productivity of oversight teams.
This kind of fast, relentless innovation is moving quickly for everyone. McKinsey reports that some executives are moving “20 to 25 times faster than they thought possible on things like building supply-chain redundancies, improving data security, and increasing the use of advanced technologies in operations.” Wow! This rings true based on the conversations we’re having with our expert advisory council and our clients as well.
The time is now for researchers, sponsors, and CRO’s to have deep, meaningful conversations about how digitally enabled productivity gains are one of the imperatives of the new normal.
2. CLINICAL TRIAL OVERSIGHT TREND #2: DATA SECURITY & INTEGRITY IS CRITICAL
As teams adopt new technology, data security and integrity is always top of mind. With new technology comes new rules and protocols all around. The amazing thing about this trend is that adopting mission-aligned smart software for clinical trial oversight will actually address productivity and data security at the same time.
This means that adopting forward-thinking technology is part and parcel to adopting greater security. It also means that making these decisions is something that leadership needs to start thinking and talking about as part of conversations with every stakeholder involved.
Whatever technology got clinical trial oversight this far is not necessarily equipped to help it grow into the future that is already here. The time for innovation and forward-thinking is here.
3. CLINICAL TRIAL OVERSIGHT TREND #3: NEW STUDY PROCESSES MATTER
The speed and efficacy with which the clinical trial ecosystem has developed vaccines and treatments for COVID-19 has been a marvel to watch. Clearly our clinical trial industry is able to move quickly and make incredible things happen while maintaining safety and oversight when we need these things most. McKinsey gives it to us straight: “The COVID-19 crisis has created an imperative for companies to reconfigure their operations—and an opportunity to transform them. To the extent that they do so, greater productivity will follow.”
Now the challenge is how to implement this kind of efficiency and productivity into the way all clinical trial oversight happens. How can we operate at our best every day and on every study? How can smart software support the highest and best use of the minds and efforts of our best science? How can automation and better, smarter processes help make great things happen?
This is our task moving forward, and a critical trend shaping the next moves of the clinical trial oversight ecosystem.
--
Whatever we were certain about a mere 2 years ago, it no longer holds true. Digitally enabled productivity and smarter remote work are the new normal, taking the clinical trial oversight ecosystem ever further.
We’re ready to talk to you about the future of your clinical trial oversight.
3 Ways Smart Technology Improves Multi-site Clinical Trial Oversight
Large, multi-site trials are an important part of clinical trial research. They allow for deeper and broader research, while increasing the number of data points. Still, the scale of a multi-site clinical trial can, in turn, scale up the administrative and oversight challenges right alongside these benefits. We look at three key challenges for multi-site clinical trials, and how using smart, cloud-based technology for managing their oversight can improve effectiveness and impact.
Large, multi-site trials are an important part of clinical trial research. They allow for deeper and broader research, while increasing the number of data points. Still, the scale of a multi-site clinical trial can, in turn, scale up the administrative and oversight challenges right alongside these benefits. We need only look to the Journal of Clinical Trials or industry whitepapers to see that challenges like communication, process, and data integrity are ubiquitous and ongoing.
Today, we look at three key challenges for multi-site clinical trials, and how using smart, cloud-based technology for managing their oversight can improve effectiveness and impact.
3 Ways Smart Technology Improves Multi-site Clinical Trial Oversight
1. COMMUNICATION
Instant, reliable communication is paramount to support quality clinical trial oversight, and this challenge is multiplied when there are multiple sites. Those at the site level are dealing with tremendous communication challenges already, and those challenges have the potential to bubble up to the oversight level. The DMC must be aware of these challenges and have tools to properly monitor the communication -- and have good tools and processes for their own internal communication.
Indeed, the DMC needs its own robust, secure, real time platform for communication -- especially in the event of communication challenges or breakdowns outside of their control. For communication to keep up with a multi-site clinical trial, the oversight committees need a single, central, and robustly secure software hub.
Smart software creates a channel for better communication.
2. DATA INTEGRITY
We know that for any multi-site clinical trial, there are many different software solutions managing many different things. This can be a source of challenges for the study. The Journal of Clinical Trials writes that “Researchers conducting clinical trials in multiple clinical sites with targeted populations face significant management challenges to maintain data integrity…” Oversight committees must be mindful of these challenges as they are reviewing data themselves and must ensure that the method and manner in which data is served to them maintains integrity, security and timeliness in order to safeguard patients.
Creating a single, central hub for the oversight of data integrity provides a reliable channel for all data, whatever its source, to be checked in a standardized, formalized way. Even if patient data is being collected in different ways by different platforms and different people, it is being checked and overseen by a single, central set of people on a single, central platform. This reinforces the data integrity of every step of the clinical trial.
Data depends on a single home for efficient oversight.
3. STANDARDIZATION
Oversight standardization is equally critical. With a multi-site clinical trial, the oversight committee is dealing with the added challenge of standardizing the oversight across all sites. This can run into challenges if reporting information or processes is not standardized, or if protocols vary across sites.
A recent whitepaper from Medidata calls out complexity of project management and collaboration as a major challenge for everyone in the clinical trial ecosystem, and added complexity means that the time and energy it takes to maintain proper oversight will increase in kind.
The answer? Standardization of oversight processes and protocols. And while this answer may feel straightforward, it also shines a bright light on how integral a single, secure software hub can be for clinical trial oversight, especially in a complex multi-site study.
Smart software supports smart oversight standardization.
Do your clinical trial oversight teams have the right tools for efficient, effective oversight?
Click here to access our essential guide to the future of clinical trial oversight
Choosing Clinical Trial Oversight Software? Here's What To Look For
Choosing the right clinical trial oversight software is critical. We need only glance at the damaging news cycles around vaccine development to see how high the stakes are and how slim the margin of error really is for communication, security, and speed.
But, what is clinical trial oversight software? Why is it so necessary? And what distinguishes great clinical trial oversight software from the pack?
Choosing the right clinical trial oversight software is critical. We need only glance at the damaging news cycles around vaccine development to see how high the stakes are and how slim the margin of error really is for communication, security, and speed.
But, what is clinical trial oversight software? Why is it so necessary? And what distinguishes great clinical trial oversight software from the pack?
CLINICAL TRIAL OVERSIGHT SOFTWARE MUST BE SIMPLE.
Clinical trial oversight software needs to create less work, not more. It needs to be simple to learn, simple to use, and simple to roll out. This means a great user interface and experience, plenty of helpful documentation, and ongoing support for administrators. Simplicity also means there should be less work for the admin because of the software, and less to remember for committee members, too. Look for smart software that uses automation.
CLINICAL TRIAL OVERSIGHT SOFTWARE MUST BE INTEGRATED.
In order to be a true one-stop-shop for oversight committees and administrators, clinical trial oversight software must be an enterprise grade integrated system that plays well with others. It must integrate with relevant clinical trial software that provides critical inputs to the work of the committees, such as eClinical data systems. It must also integrate with systems that rely on oversight committee information, such as eTMF software. Finally it must provide or integrate with standard productivity solutions such as document, signature, meeting and video conferencing management software. These integrations are powerful drivers of security, speed and efficiency in oversight work and communications.
CLINICAL TRIAL OVERSIGHT SOFTWARE MUST BE SECURE.
When we say secure, we mean airtight security that meets the highest possible standards. Truly secure clinical trial oversight software should meet the standards of a SOC 2 certification, use the highest level of data encryption, provide intrusion detection and breach monitoring, offer 24 hour continuous monitoring, and of course have protocols like access control and two-factor authentication. Patching together productivity software plus regular email and video chat is not secure enough to manage clinical trial data or communications. Great clinical trial oversight software will reliably protect unblinded data and reduce confusion in favor of clarity every step of the way.
CLINICAL TRIAL OVERSIGHT SOFTWARE MUST BE FLEXIBLE AND SCALABLE.
Finally, clinical trial oversight software must flex to fit your operations in terms of scale and process. It should not only scale as your needs grow, but it should provide the operational leverage that allows your teams to do more. It should serve two or twenty members equally well, and also enable administration of one hundred studies as efficiently it does one. Key elements to look for include easily customizable, automated workflows that empower administrators and software with a development team who are consistently innovating and adding features in response to your needs.
Are you ready to kick your search for the best clinical trial oversight software into high gear?
Click here to access our essential guide to the future of clinical trial oversight
5 Signs Your Current DMC Software Is Outdated
Have you done a review of your DMC software lately? Has a review ever been done? Do you know who is responsible? Now is the time.
As Business2Community points out, using outdated software can cause a range of major problems, risking data loss through software failure, exposing your work to software bugs or cybercriminals, and leaving important work open to lost productivity through inefficiencies or sudden software incompatibility. The list goes on.
We need only glance at Harvard Business Review to remind ourselves why “In the Digital Economy, Your Software is Your Competitive Advantage.” This is true across industries, and has become an urgent, necessary need for every stakeholder in the clinical trial oversight ecosystem.
Is Your DMC Software Outdated?
Here are 5 signs to look for.
1. YOUR DMC SOFTWARE IS ACTUALLY MANY SOFTWARES COBBLED TOGETHER
Does your data monitoring committee use integrated software? Or, like most teams, are you patching together email, texting, video calls, and a variety of partial security measures that aren’t designed to work together?
If your DMC software isn’t a single, secure hub, you are open to all kinds of security threats and inefficiencies.
2. YOUR DMC SOFTWARE MAKES ONBOARDING DIFFICULT
When you think about onboarding new members to your DMC, or writing a charter and starting with an entirely new data monitoring committee, how does your gut feel? Do you have that moment where you realize the new people will need to create multiple new accounts and learn new habits just to get started?
DMC software that is all inclusive and holds everything a monitoring committee needs in one place is truly the only way to go in today’s asynchronous, remote working environment -- and it makes onboarding a snap.
3. YOUR DMC SOFTWARE IS SECURE… YOU THINK?
Who on your team can vouch 100% for the total and complete security of all data and communications related to your data monitoring committee? Not sure who? Not sure what that would even mean?
That means your software and/or your process are dangerously outdated. It’s time to look for a comprehensive solution that is verified to the highest industry standard.
4. YOUR DMC SOFTWARE DOESN’T EXPORT THE FILES YOU NEED
It’s no secret to any DMC what files and data that sponsors, CROs, AROs, or even the FDA will need to review. So if the software your DMC is using doesn’t set everyone up for success by supporting the creation and tracking of those files, then how is it really helping you?
Great, smart software is available and has come of age. If your software doesn’t export the files you need, it’s time to find one that does.
5. YOUR DMC SOFTWARE DOESN’T MAKE YOUR ADMIN’S LIFE EASIER
In the end, clinical trial oversight software should benefit everyone in the clinical trial ecosystem. But the DMC admin needs to love the software the team uses. If the admin isn’t saving time and seeing benefits to stakeholders every step of the way, why are you using it?
BOTTOM LINE: DMC software should deliver speed, scale and security, making everyone’s life easier.
Ours does.
Click here to explore why Cloud Concinnity is the best way
to update your DMC software.
Beyond COVID: 3 New Standards for Clinical Trial Oversight
The Covid pandemic has impacted the entire clinical trial oversight ecosystem. What worked just a year or two ago will no longer get the job done. Clinical trial oversight committees need a new way to address complicated challenges like working remotely and asynchronously, and they need the right software to get things done efficiently and effectively.
The Covid pandemic has impacted the entire clinical trial oversight ecosystem. What worked just a year or two ago will no longer get the job done. Clinical trial oversight committees need a new way to address complicated challenges like working remotely and asynchronously, and they need the right software to get things done efficiently and effectively.
It is time to be creative and innovative about how clinical trial oversight work is done so that it keeps pace with the rapid changes, pressures, and variabilities that are now part of clinical trial management.
Those who want to thrive in clinical trial oversight beyond Covid need to engage at a high level of speed, scale and security.
Here are the three new clinical trial oversight standards that we see as mandatory to thrive in the world beyond Covid -- and how we’ve already integrated these oversight standards into Cloud Concinnity so that forward-thinking teams who use our software can be sure they are prepared by default for the new normal.
The New Standards for Clinical Trial Oversight in 2021
1. FACILITATED ACCESS
Covid has changed how we communicate, and forever made instant communication the new normal. Oversight committees are expected to communicate much more often than in the past, and be able to access both data and each other anytime, anywhere.
The dynamic study ecosystem of today asks oversight committees to respond in real-time to new developments, news cycles and other stakeholder demands. Where traditional timelines saw periodic meetings to review data, the new normal demands 24/7 access and availability.
his new cadence means teams need to be more adaptive. The new nature of oversight calls for insight and innovation. We must put systems in place where all oversight committees and other key leaders can easily and securely communicate insight to sponsors and CROs.
Cloud Concinnity does this with a single, secure hub that lives in the cloud. Wherever any committee member is, they can access data and each other as long as there is an internet connection. As an example, we prioritize secure online messaging and video chat, because that is how people work now.
At the same time, this access must be carefully facilitated to ensure security for patient information, aggregate data, and communication records. Sponsors and CROs need easy-to-use, strict controls over who sees what data, who has access to what documents, and how information is stored.
In short, you need facilitated access, not simply open access. That’s why Cloud Concinnity offers detailed, role-based permissions to allow deep customization.
Click here to learn more about how Cloud Concinnity offers facilitated access.
2. CONTROLLED PROCESS
Just as Covid has changed where people work, it has also changed how people work.
In-person meetings and a reliance on printouts are out, while online meetings and Zoom calls are in. We built Cloud Concinnity on this kind of cloud-based communication -- with the flexible and controlled process to go along with it.
The pandemic has already disrupted the entire clinical trial ecosystem, delaying or canceling almost everything. Even now, it continues to hamper, slow or stall trials for all kinds of new drugs and treatments. For any oversight committee dealing with a Covid-related drug trial, the pressure to move quickly is high.
The only way for those trials to catch up is for the entire clinical trial oversight ecosystem to get more efficient, and the only way for that to happen is to create consistent, controlled processes that will change the way things are done.
While some teams are dipping their toes into digitizing reports or using productivity apps, the only solution that solves the problem of how to effectively control processes is using a single, secure hub like Cloud Concinnity.
A shocking amount of time is attributable to administrative work at a time when such resources are increasingly scarce. This can be eased and minimized with smarter technology and automated processes.
The new standard for oversight tech is to automatically track who has done what and when, making the work of admins easy, efficient and trackable.
At the same time, committees and their work are subject to increasing scrutiny through regulatory pressure, auditing, and media attention. A platform that ensures, and documents, regulatory compliance and implements best practices is the tool of choice for those responsible for ensuring and overseeing compliance.
Cloud Concinnity does all of this, and is built around controlled process. Automated processes mean more efficient interactions, consistent application of best practices and significantly reduced risk of delays, errors and noncompliance.
Click here to learn more about how Cloud Concinnity supports controlled process.
3. TRANSPARENT OUTCOMES
Finally, Covid has also changed what we all expect from each other and from our work.
Not only do we expect quality, speed and scale from our businesses, but we expect flexibility and transparency from each other. Covid has pushed us all to become more adaptive and more creative.
And to do that, we need reliable data to share with each other, and visibility to build insight on top of. Today’s clinical trial oversight committee members need reliable insight into what matters.
This means a new level of transparency and accountability for all stakeholders in the clinical trial ecosystem. It means regularly updated, agreed upon performance metrics.
And it means using software that offers a central dashboard, reliable analytics, workflow progress reports and audit-ready information all along the way.
Faster insights and faster action come from smart software that facilitates access for teams, allows admins to control processes, and wraps all of this innovation together with reliable, transparent outcomes.
Cloud Concinnity is always audit-ready and simple to export, interactions are automatically documented and available for review anytime, and everything is always up to date.
Click here to learn more about how Cloud Concinnity delivers transparent outcomes.
IN CONCLUSION
Thriving in today’s clinical trial oversight ecosystem is not easy. But it is possible, and it starts by understanding the new standards for clinical trial oversight.
Is your oversight committee strategy ready for the world beyond Covid?
5 New Ways Clinical Trial Oversight Practices Can Rise To Today’s Challenges
Today’s turbulent environment has precipitated an unprecedented level of pressure on sponsors, CROs, and the ecosystem of oversight committees needed to create and conduct a clinical trial. The stakes are simply higher now for every stakeholder involved in funding, managing and overseeing clinical trials.
Today’s turbulent environment has precipitated an unprecedented level of pressure on sponsors, CROs, and the ecosystem of oversight committees needed to create and conduct a clinical trial. The stakes are simply higher now for every stakeholder involved in funding, managing and overseeing clinical trials.
The accelerated rate of change means that if these key stakeholders aren’t effectively engaging key issues of strategy and risk, they are competitively handicapped at best and, at worst, in risk of crises.
Taken together, these factors pose a serious threat to traditional practices of clinical trial oversight. Here are some of the old processes that are being challenged -- and how Cloud Concinnity can facilitate critical transformation.
1. ENGAGEMENT CADENCE
Clinical trial oversight historically followed a predictable, steady cadence of infrequent meetings. Certainly, there was no way for oversight committees to be in daily contact. Events that once evolved slowly now move much more quickly. Also, risks around safety and efficacy events aren’t so predictable.
Without platforms and processes in place to ensure oversight teams can communicate and engage outside the typical meeting cycle, leadership’s contribution becomes reactionary rather than proactive.
Smart, cloud-based software means that oversight committee members can stay updated and engaged anytime from anywhere. It’s the pace at which trials move, and the pace at which oversight must as well.
2. INFORMATION EXCHANGE
Many oversight committees are still relying on a multitude of sources and download points for information and documents to send information between sponsors, CROs, and oversight committees. An issue arises when such a glut of information is provided all at once, or when there is a sudden issue calling for action and comment and everyone involved needs to be on the same page right away. The predictable information asymmetry and lack of transparency will inevitably cause problems.
In short, if the committee is not regularly sharing information with each other, there can be a serious time lag for everyone to get up to speed and make important, urgent decisions that are well-informed.
We need only glance at today’s headlines to see what happens when clinical trial information is out of sync. This is the old playbook at work. An efficient method of information exchange is essential for everyone on the team to regularly share uptodate information with one another. It means all decisions can be better-informed decisions.
3. MANUAL PROCESSES
Clearly clinical trial speed and efficiency is more important than ever -- and requiring more time than ever. So why aren’t oversight committees equipped with the best task management tools to automate mission-critical processes?
While members can cobble together multiple tools for a small shot in the arm to efficiency, when they are not a single, secure hub enclosed in a protective layer of security they become more part of the problem than the solution. Automating what are typically manual processes adds a tremendous amount of value and makes the single hub a vehicle of tremendous efficiency.
4. TRANSPARENCY
Increased pressures have made it even more critical to quickly anticipate challenges and course correct accordingly. Sponsors and CROs have a responsibility to empower oversight committees to see and drive these corrections, and every stakeholder in the clinical trial process is increasingly under the microscope for its ability to do so effectively.
Without on-demand access to regularly updated data and performance metrics, stakeholders find it difficult to effectively fulfill their responsibilities. Cloud Concinnity's software is unique because it facilitates updates for all users in real time. Transparency is achieved through efficient information sharing.
5. RESISTANCE TO CHANGE
This isn’t a nuanced challenge: the fact is that many sponsors, CROs, and oversight committees are simply resistant to a new way of doing things.
With the rate of change in the clinical trial ecosystem skyrocketing, an unwillingness to keep pace puts patient health, study integrity and value creation in extreme jeopardy.
What’s more, in a competitive marketplace, it is the innovators who differentiate themselves and their market positioning, not those who are using the old playbook.
Cloud Concinnity allows stakeholders to be clear forward-thinkers and innovators in clinical trial oversight.
CONCLUSION
In today’s turbulent environment where sponsors, CROs, and the ecosystem of oversight committees are under immense pressure, the stakes are high for funding, managing and overseeing clinical trials. Old processes and playbooks must be challenged -- and changed.
Cloud Concinnity can facilitate the critical transformation and help clinical trial oversight rise to the many challenges of today.
Learn More About How Cloud Concinnity Can Improve Your Oversight
4 Critical Takeaways from AstraZeneca and its DSMB
The news that AstraZeneca's Data Safety and Monitoring Board (DSMB) has publicly challenged the company’s drug efficacy reporting reveals a clinical trial oversight system pushed to its limits. Here's what you need to know and take away from the situation.
News that a Data Safety and Monitoring Board (DSMB) overseeing AstraZeneca’s Covid-19 vaccine trial publicly challenged the company’s drug efficacy reporting has been a gut punch to the entire clinical trial ecosystem.
In a world clamoring for faster and faster vaccine development, we are seeing spectacular success in getting life saving drugs to market. We are also seeing a system pushed to its limits. Developing multiple safe, effective vaccines in under a year is incredibly impressive. Distributing hundreds of millions of doses is equally amazing. At the same time, the stress and pressure of moving quickly while also grappling with rapidly evolving communication requirements and procedural adaptations has introduced a host of new challenges at every point in the drug development process.
Critical independent trial oversight, the kind provided by DSMBs, historically progresses over months and years. That luxury of time to act, react and communicate more deliberately has disappeared.
Whatever the multiple and varied root causes of the current clinical trial crises are, they shine a bright light on what’s critical and essential in good clinical trial oversight. We are seeing in real time just how important DSMBs are. We are also seeing that communication and process norms that have worked for decades need to evolve, change, and adapt to the new normal.
Every clinical trial stakeholder who is involved in the regulatory and independent oversight committee processes, from sponsors, to CROs, to DSMBs, can seize this moment to look at communications, processes, and systems.
1. TAKE A HARD LOOK AT COMMUNICATIONS
It’s not the people. It’s the processes. It’s the systems.
The vast majority of people in the clinical trial ecosystem — researchers, sponsors and independent bodies — approach their work with high scientific and ethical standards.
Mistakes can easily happen when processes and systems for coordinating and documenting oversight are stretched too thin, or when oversight tools are decentralized. Today most DSMBs are forced to rely on processes and systems for creating transparency, communications and reporting that are fragmented inside and outside of their organizations. Administrators can find themselves at the center of rules, communications and processes that are not optimized for speed and efficiency and increase risk for non-compliance or miscommunication.
Without an integrated set of information and communication tools, it’s impossible to know the status of oversight committees or to audit effectiveness in uncovering research issues or unintentional process violations. It’s a real threat to otherwise tight clinical trial processes.
2. CONSIDER HOW STANDARDIZED PROCEDURES IMPACT TRUST & SAFETY
Standard Operating Procedures (SOPs) have a critical role to play for DSMBs and the overall process of clinical trial oversight. Whether born of the charter and study protocol, the safety plan, or other documented sources, creating and following SOPs is the road to safe, speedy trials that inspire trust.
The challenge facing DSMBs and administrators is not so much knowing or developing these operational best practices, but implementing, tracking and reporting on them. Too many studies are still relying on manual processes or using multiple software tools that aren’t designed to work together. The results of these kinds of disconnected solutions are not pretty, as we are seeing.
The depth and breadth of these challenges demand simplification, centralization and automation. What is needed is a powerful process management engine that can drive an integrated process and provide visibility into current status and historical actions.
What’s needed is smarter technology to provide a process management backbone for the independent oversight mechanisms. The future success of clinical trials is dependent on digital transformation. It’s time for the oversight part of the clinical trial system to be supported by software as powerful as the oversight committees themselves.
3. SUPPORT YOUR OVERSIGHT COMMITTEES WITH SMART SOFTWARE
We believe in the power of smart technology to transform mission critical processes in service to a better world. Solving communication and process problems for oversight committees like DSMBs is our mission.
When we first launched Cloud Concinnity® as a governance, risk and compliance platform, it was used to solve a range of board and specialty governance management challenges. When COVID upended the world, it became the ultimate communication and process engine for DSMBs. We are passionate about helping DSMBs ensure patient safety while bringing life saving drugs to market.
Cloud ConcinnityⓇ takes the work of an oversight committee and creates repeatable best practices, then instantly reports the status of the work and provides a comprehensive audit trail.
Think of Cloud ConcinnityⓇ as an eClinical solution built on a robust, integrated, cloud based governance process management engine. It provides a single, secure hub to organize, coordinate and document the end to end activities of the DSMB.
Whatever the appropriate processes are for any trial oversight committee, here is how Cloud ConcinnityⓇ helps improve the execution, documentation and visibility of SOPs:
SOPs for an oversight committee are broken down into tasks
Tasks are assigned to individuals by their roles
Execution of the tasks is tracked
All actions within the system are logged
Regular reporting of SOP status is visible as desired
Comprehensive audits are available in seconds, since all work is in Cloud Concinnity
4. ACT NOW
Today’s flurry of news is not just an urgent, pressing problem for the current round of vaccine development — it is a clarion call for every stakeholder in the clinical trial ecosystem moving forward.
Imagine the improved communications for DSMBs that begin using a single, secure cloud based hub to communicate. Imagine how clear and strong that message about the culture of respect and support for DSMBs will be sent. Imagine how sponsors can step up right now and show how fully they support their DSMBs by providing smart technology.
The problems flooding the news right now are preventable for those ready and willing to take action. Smart software solves communication problems. SOPs support safer, more trustworthy clinical trials.
We have the solution, ready to be deployed.
To learn more, explore our DMC & DSMB software.
For details, check out our Features & Benefits document.