Transforming Clinical Trials’ Coordination Through Automation
Discover how automation in clinical trials tackles delays and inefficiencies by streamlining data management, enhancing collaboration, and ensuring regulatory compliance for independent safety review committees. Learn how this shift optimizes resource use, accelerates time-to-market, and supports more reliable trial outcomes
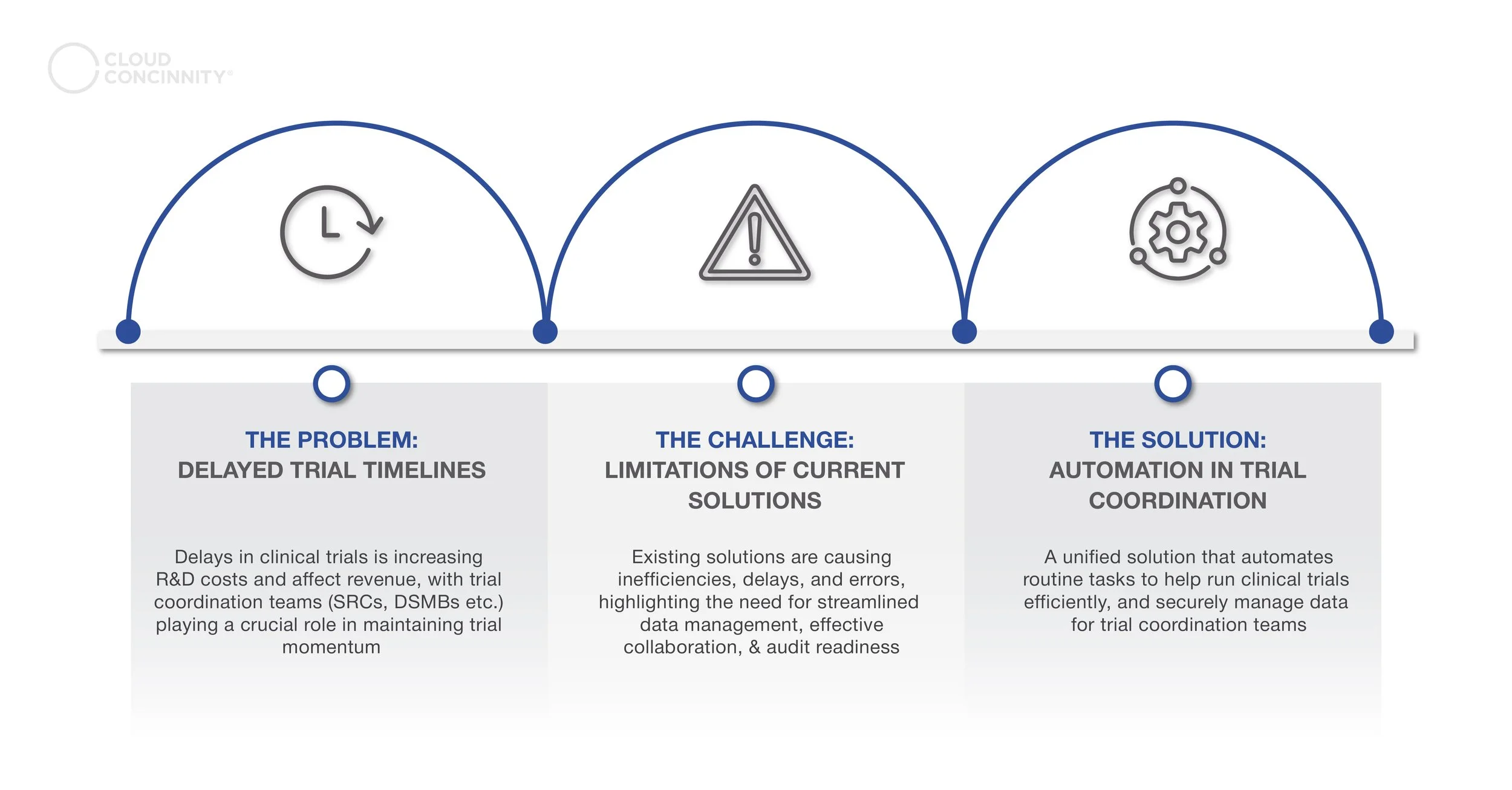
Clinical trial delays are a common challenge in drug research and development. Eight out of ten trials experience setbacks, with around 94% of these delays lasting longer than a month. This often results in increased costs, reduced profitability, or, in some cases, project failure. The root causes of these delays are frequently linked to unmet or complex regulatory compliance standards, gaps in procedure management, inefficient processes, and communication breakdowns between teams.
While it’s impossible to account for all unexpected challenges in today’s complex research environment, there are effective strategies to minimize the likelihood of clinical trial delays. One key approach is the integration of automation into clinical trial management.
Clinical Trial Coordination Committees play a vital role in ensuring patient safety and the scientific validity of trials. However, the current trial coordination landscape is complex, involving numerous stakeholders, intricate processes, and large volumes of data, all of which can negatively impact efficiency and contribute to delays.
Transforming Clinical Trials’ Coordination Through Automation - Problem , Challenge and Solution
The rise of automation technologies is reshaping clinical trial coordination. Traditional trial processes, which involve complex workflows managed by multiple teams, often lead to inefficiencies, delays, and errors. Automation simplifies these workflows by reducing repetitive tasks and centralizing operations, making trial management smoother and more streamlined. This shift not only enhances data quality but also helps detect unexpected variability that could affect trial outcomes. As a result, automation optimizes resource use, accelerates time-to-market, and alleviates staff workloads, ultimately making the clinical trial process more efficient and reliable. Many of the daily challenges faced by teams, such as independent safety review committees, can be alleviated with an automated solution. Here are the top five challenges experienced by clinical trial coordination committees:
1. Centralized Data Management: Clinical Trial Coordination Committees must carefully manage both blinded and unblinded data to avoid mistakes. Managing multiple committees across different trials, and accessing safety data without a unified platform increases the risk of data leaks and compliance issues, which can lead to inefficiencies and delays. Centralization of this process will help streamline both data management and trial coordination.
2. Document Management and Collaboration: Producing, sharing, and storing standardized documents like charters, agendas, and minutes can be challenging. Without a centralized system, documents become scattered, causing inconsistencies, while relying on emails or traditional repositories makes collaboration cumbersome and time-consuming.
3. Audit Readiness and Compliance: Achieving audit readiness and maintaining compliance requires detailed documentation and frequent updates. Regulatory teams must adhere to specific FDA and Charter requirements for each study. Clear eTMF documentation of Safety Listings, Document Distribution, and documented decision-making (eg. recommendation letters), is also essential to ensure all processes are accurately recorded and readily accessible for audits, a task that is challenging without the right platform.
4. Communication and Task Management: Organizing Trial Coordination Committees’ meetings across time zones is challenging and time-consuming without centralized scheduling tools, leading to delays and poor communication. Coordinating with statisticians for reports and sharing committees’ decisions throughout a trial requires strict procedures, and without the right tools, there's a real risk of things getting off track.
Addressing these challenges requires a decisive move toward automation. Automating routine tasks helps committees manage information more securely and run trials more efficiently. Cloud Concinnity has identified three key areas where automation can enhance the efficiency of clinical trial coordination:
1. Centralization: Cloud Concinnity’s Global Dashboard consolidates all projects into one hub, eliminating the need for multiple platforms. It provides secure, role-based access, centralizes key documents, and offers automated task alerts. Efficient safety listings and document distribution save time, while streamlined account management simplifies platform access.
2. Collaboration: Cloud Concinnity enhances collaboration with real-time document editing for faster revisions and accelerated approvals. It ensures secure teamwork through controlled access, automates access transfers during personnel changes, and integrates with Adobe Sign & DocuSign for easy, compliant signing.
3. Compliance: Cloud Concinnity ensures audit readiness with its Regulatory-Ready eTMF Integration. It streamlines sponsor audits with efficient access controls and customizable workflows to meet FDA requirements, while visualized tracking keeps committees on top of regulatory needs.
Automation has become a necessity in healthcare, especially in clinical trials where speed and safety are paramount. By streamlining processes, automation overcomes the inefficiencies of traditional methods, driving operational success.
To effectively incorporate automation in clinical trials, it's crucial to identify the right tasks for digital transformation. Processes such as document management, signal detection, and safety reviews are ideal for automation, as they are data-heavy and involve repetitive work. This approach not only improves efficiency but also enhances customer benefits and addresses specific operational problems, ultimately driving better trial outcomes and regulatory compliance. Effective automation requires not only the right tools but also staff education. Training should enhance digital literacy, helping teams identify automation opportunities and focus on higher-value work.
Automation is not just transforming clinical trials—it's reshaping the future of patient safety and operational excellence, ensuring that life-saving treatments reach the market faster, safer, and more efficiently than ever before.
Learn more about Cloud Concinnity® and schedule a demo today.
5 Clinical Trial Trends You Need to Know in 2023
Here are five trends that clinical trial managers must be aware of in the clinical trial space to maximize performance and improve the likelihood of a successful clinical trial outcome.
As 2022 draws to a close, one observation about clinical trials continues to ring true. Clinical trials are becoming more complex, and sponsors require more from their sites. Clinical trial managers must be aware of the latest trends in the clinical trial space to maximize performance and improve the likelihood of a successful clinical trial outcome. Below, we've compiled a list of the five clinical trial trends that you need to keep front of mind in 2023:
Five Leading Clinical Trial Trends in 2023
The Continued Rise in Digital Clinical Trials
As technology advances, so do the inclusion of these new technologies in managing clinical trials. More and more sponsors require that sites utilize digital technologies in their studies, from electronic case report forms (eCRFs) to cloud-based data capture systems.
Technology can only help streamline the trial process by reducing paperwork, keeping study teams up to date on the latest developments at all times, and eliminating costly errors.
This need for clinical trial oversight-specific software is why we created the Cloud Concinnity Platform. With Cloud Concinnity, your clinical trial managers and employees can quickly produce, share, and store critical clinical trial process management, data, and communications - all from the security and convenience of the Cloud. Learn more about Cloud Concinnity here.
Aggressive Trial Timelines
The global pandemic, supply chain issues, and the rise of inflation have caused clinical trials to be delayed for the past several years. To make up for this lost time, sponsors will be pushing out aggressive clinical trial execution timelines that require sites to work even harder than before to meet tighter deadlines.
Sites that can quickly adapt and keep pace with these increased demands are sure to be successful in the future. However, areas that need to adapt and maintain efficiency in an increasingly competitive climate may be left behind.
One crucial way for sites to stay ahead of the curve is by investing in new technologies and systems that can help them streamline their operations, improve patient recruitment efforts, and automate regulatory compliance more easily. For example, implementing electronic data capture can help sites speed up trial execution times by reducing paperwork and minimizing errors. Likewise, using a system with automation and workflows can help to standardize practices at scale. It also empowers your team with the ability to automate their workflows.
The Rise in Trial Consolidation
As clinical trials become more complex, sponsors will begin to look for ways to consolidate their studies and streamline processes to reduce costs and improve efficiency. Sites offering a full range of services across multiple indication areas will be highly sought after by sponsors as they work to consolidate their studies.
There are several reasons why clinical trial consolidation is becoming a popular choice in 2023.
The number of interventional trials grows, sponsors will look for ways to simplify their processes.
Sponsors may also be interested in reducing costs by consolidating studies into fewer sites to more efficiently manage their budgets.
Finally, with sophisticated data analytics capabilities and a growing network of clinical trial sites, sponsors will have no trouble finding the right location for their studies.
Sponsors looking to consolidate their trials in 2023 should choose sites that offer a full range of services across multiple indication areas. For example, some sites may be specialists in oncology or other specific diseases, while others may have more experience with drug development in general. By choosing a site that can offer multiple services, sponsors can reduce their overall costs and improve efficiency.
More Aggressive Screening Practices
To increase trial efficiency and improve data quality, sites will be required to screen patients more aggressively than before. This means that study teams must have efficient screening methods and invest heavily in technology solutions designed to support these procedures.
They must also have protocols in place that encourage engagement between the site, the patient, and the sponsor. This way, sponsors can be confident that trial data has been obtained from patients with a high likelihood of responding to treatment.
Trend Toward Personalized Medicine
With personalized medicine emerging as a major trend in healthcare, clinical trials are quickly adapting to this new approach. By understanding each patient's unique genetic makeup, sites can create more targeted interventions that improve outcomes and reduce the burden placed on patients during the study process.
By reducing unnecessary and duplicative trials, personalized medicine has the potential to save time, money, and resources for the broader healthcare industry. However, some barriers still need to be overcome before this model can fully take root in clinical trials.
For starters, much of the technology needed for personalized medicine is still nascent compared to other areas of healthcare delivery. The big data analytics required to reliably interpret patient DNA or genomics data is not yet readily available at all sites conducting research. In addition, sites that have access to this type of technology require highly specialized personnel – including genetic counselors, bioinformaticians, and biostatisticians– who are in high demand across many industries. As such, it can be challenging to recruit and retain these professionals, particularly if they are being asked to take on additional study sites or trials.
As these trends continue to emerge in the years ahead, sites need to stay up-to-date on all the latest developments so they can better prepare for what lies ahead. To learn more about how you can future-proof your clinical trial operations and thrive in 2023, be sure to schedule your Cloud Concinnity demo today!
5 Ways to Streamline Multi-Site Clinical Trial Management
Clinical trials are complex and expensive endeavors that require researchers to track enormous amounts of data, manage multiple stakeholders/participants, and communicate efficiently - all while following protocols. And that's just single-location trials!
But what happens if your clinical trial needs to run across many different sites? Then all of this complexity increases substantially, so you'll need to streamline your multi-site clinical trial processes and procedures to ensure it is completed on budget and schedule. Use our tips below to improve your multi-site clinical trial administration.
5 Ways to Streamline Multi-Site Clinical Trial Administration
Use a Centralized Cloud-Based Oversight Tool
Automate Processes Across Sites
Improve Information Sharing and Communication Efficiency
Systematize Your Outcome Tracking
Stick to the Schedule
Use a Centralized Cloud-Based Oversight Tool
One of the easiest ways to improve multi-site clinical trial administration is to ensure that you have a single source of truth for the aggregation of information from each of your locations. This enables real time oversight of protocol adherence and results tracking back oversight teams, from safety committees to DMC to sponsor and CRO administrators. Ideally, invest in a cloud-based system that can be accessed by approved individuals from any location that communicates with or replaces other tools. This will provide your team with a centralized system for managing communications, processes, and reporting.
Pro-tip, a software like Cloud Concinnity can help in this regard. Our cloud-based platform was built to meet the specific needs of clinical trial oversight committees.
Automate Processes Across Sites
Introducing automation into your multi-site clinical trial administration is a must for improving the operational efficiency of multi-site trials. With automation, you can eliminate many of the manual aspects of clinical trial administration - scheduling meetings, sharing notes, creating and approving required documentation, etc. Automation can also help to eliminate errors, reduce the risk of delays and noncompliance and ensure standardization.
Improve Information Sharing and Communication Efficiency
With many different clinicians and researchers across the sites, a strong communication platform is critically vital in efficiently administrating your multi-site clinical trial. Researchers will need to be able to communicate with each other easily and quickly. They’ll also need to be able to share and access critical data files effortlessly.
This is where using the right tool can make all the difference. Make sure your communications and confidential data are being transmitted across secure, Part 11 compliance systems.
Systematize Your Outcome Tracking
Outcome reporting can be a significant time investment in multi-site clinical trial administration. To improve your management efficiencies, we recommend systemizing your outcome tracking and reporting. Work with all of your different locations to ensure that they are reporting outcomes in a consistent manner - one that can easily be fed into your reporting frameworks.
And make sure that those outcomes make it all the way from multiple sites to the critical safety and oversight teams charged with deciding on trial progression. A system like Cloud Concinnity can help to streamline your outcome reporting, tying outcomes and communications around them to multiple systems and locations in real time.
Stick to the Schedule
Lastly, with so many different variables occurring simultaneously in a multi-site study, it can be easy to fall behind. A consistent schedule of milestones and deadlines is critical to ensure that your team stays on track.
Be sure to hold your team accountable to these expectations and implement systems to track progress against these milestones.
Ready to optimize and improve your multi-site clinical trials? We can help! Our system was built to meet the needs of your multi-site clinical trial.
Contact us today for a free demo of Cloud Concinnity to learn more about how our tool can help you streamline your next trial.
5 Tips for Standardizing Your Clinical Processes
Clinical trials require require standardized processes that drive efficiency as well as enhance safety and ensure compliance. Here are tips for standardizing those clinical trial processes.
Clinical trials are essential to the success of new medical treatments and therapies. They help researchers study the effects of new treatments in a safe, controlled environment before these treatments are made available to the wider public.
However, running clinical trials can be expensive, time-consuming, and difficult. There are many different factors to manage, data to collect, and patients to monitor. This is why it's so important to develop standardized processes. Process standardization will drive efficiency as well as enhance safety and ensure compliance as you complete your trial.
In this blog post, we’ll discuss five tips that will help you standardize your clinical trial’s processes.
1: Document Protocols
Before you begin your clinical trial, it’s important to develop clear and concise protocols. Documenting what must be done when, by whom will ensure that end goals will be met in compliance with SOPs, charters and regulators. These will be the guidelines that you and your team will follow throughout the course of the study to ensure that it’s successfully completed on time.
When developing these protocols, your team should try and account for the goals of the study and the requirements needed to complete these goals. This document should outline the objectives of the trial, the eligibility criteria for participants, and the procedures that will be followed during the study for everything from recruitment to oversight to FDA application and review.
Begin with the end in mind! By laying this foundation at the beginning and developing a protocol upfront, you will be able to avoid potential delays and disruptions during your clinical trial.
2: Mapping Processes Is Key
Mapping out your clinical processes is key to standardizing them. By understanding the steps involved in each process, you can identify where improvements can be made and what needs to be done to streamline the process.
Additionally, understanding your processes can help you troubleshoot issues that may arise during a clinical trial. There are many benefits to mapping out your clinical processes. By doing so, you can:
Improve efficiency and quality
Reduce variation in how tasks are performed
Facilitate training of new staff
Make it easier to identify potential risks
Improve compliance with Good Clinical Practices (GCPs)
3: Optimize Data Management
One of the most important parts of a clinical trial is data collection, as the data will be used to determine the conclusions that the decision makers are able to draw from the study. But how to ensure that you’re providing all of the right information to the right people at the right time?
One way is to develop standard operating procedures (SOPs) for the collection, aggregation and dissemination of information to decision makers. SOPs outline the steps that should be taken during each stage of the clinical trial, from screening participants to closing out the study. SOPs also ensure that everyone working on the study is on the same page, as the standardized procedures are widely available to everyone. This can prevent miscommunication and eliminate incomplete data collection at different points in the study.
To further improve your data analysis, the data collected in your study can be aggregated into an integrated software platform like Cloud Concinnity. This will allow for centralized access to and control of information critical to decision makers at key trial stage gates. Cloud based, integrated systems allow you to centralized your research data in one place so research teams can access it whenever and wherever needed.
4: Leverage Clinical Trial Software
Managing the various components of a clinical trial can be complex and challenging,.especially when it comes to communicating standardized processes to the broader team and holding them accountable for adhering to those standards.
To help simplify this, it’s important to use clinical trial software that actually has a process management engine at its core. Process management engines eliminate the variability inherent in manual processes by automating them. By eliminating as many manual processes or point software solution bottle-necks as possible, you can create an environment where your team is more likely to abide by a standard process. Automating clinical processes improves efficiency and accuracy saving time and money and eliminating process variability that can put your study at risk.
Cloud Concinnity is purpose built to bring good process to clinical trial oversight. It offers unparalleled advantages to anything on the market today by centralizing, standardizing & automating oversight of key trial elements. Oversight teams need a single secure platform to support decision making at key stage gates to ensure quality, efficiency, accountability and control
5: Create a Budget and Follow It
Clinical trials can be expensive and time-consuming, and it’s easy for costs to get out of hand. That’s why it’s so important to develop a budget for your trial. An accurate budget should take into account expected expenses as well as costs of similar, previous trials to incorporate a historical basis of how much the study should cost. Expenses can range from participant payments, to research supplies, to software to help you track and complete your research trial.
By detailing all of these costs in a budget before the study begins, you’ll be able to avoid potential financial problems during your clinical trial. This will help to ensure that your study is completed on time and within budget.
In summary, developing the standardized processes for your clinical trial will help you manage all of the different factors of the study. And software like Cloud Concinnity can help your team manage these factors, collect data, and communicate effectively and easily. For a free demo of Cloud Concinnity, please feel free to reach out to us today. We’re here to help.